Tailored valve interventions prove effective for LVOTO without significant septal hypertrophy
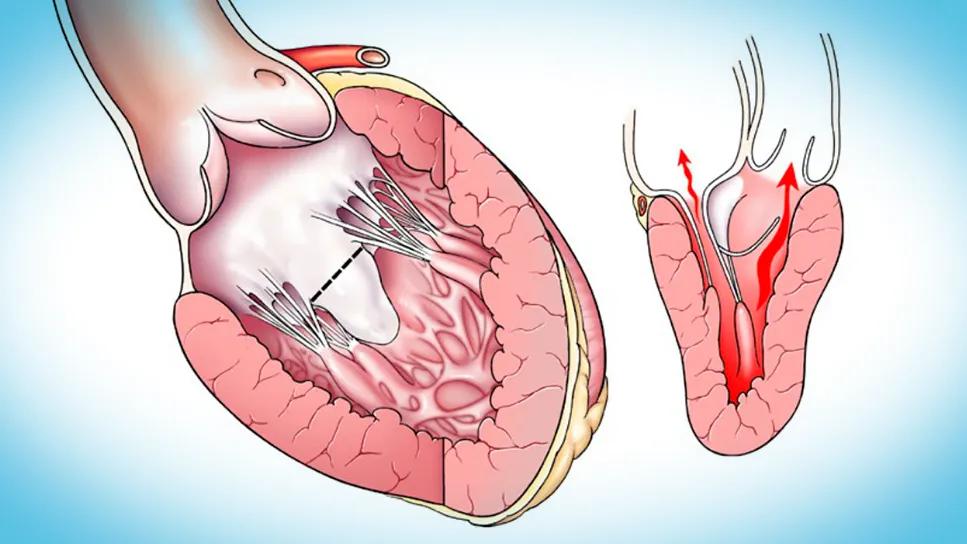
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/39580657-0c34-4a3d-985b-3eb6f5f2faa1/CQD-Mitral-valve-induced-LVOTO)
two illustrations of a thickened human heart
For decades, conventional understanding of left ventricular outflow tract obstruction (LVOTO) has centered on hypertrophic cardiomyopathy (HCM) with significant septal hypertrophy, leading to a primary surgical focus on extended septal myectomy. However, a new research paper from Cleveland Clinic underscores an important shift toward recognition that (1) mitral valve abnormalities alone can cause significant LVOTO, even in the absence of substantial septal hypertrophy, and (2) tailored mitral valve interventions offer a safe, effective and often repair-based solution in these cases.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The authors’ approach to mitral valve-induced LVOTO, which has evolved over three decades, offers what the paper dubs a “surgeon’s toolkit” of techniques to relieve obstruction, resolve mitral regurgitation (MR) and eliminate systolic anterior motion (SAM). The approach spares most patients from mitral valve replacement and the need for lifelong anticoagulation.
The new paper, authored by a team from Cleveland Clinic’s Heart, Vascular and Thoracic Institute, details the toolkit and challenges the traditional septum-centric view of LVOTO by highlighting the independent and often primary role of the mitral valve and its subvalvular apparatus. “This paper provides crucial guidance on identifying and surgically addressing LVOTO in a patient subset previously thought to be less common or for whom management was less clear,” says senior and corresponding author Nicholas Smedira, MD, MBA, Surgical Director of Cleveland Clinic’s Hypertrophic Cardiomyopathy Center.
While LVOTO is classically diagnosed in patients with HCM and substantial septal hypertrophy — where midventricular hypertrophy directs flow vortices to displace the mitral leaflets anteriorly into the LVOT —increasing numbers of patients are being diagnosed with LVOTO despite minimal or no septal hypertrophy. Cleveland Clinic’s extensive experience, encompassing over 4,000 septal myectomies in the past three decades, reveals that approximately 5% of LVOTO patients primarily require mitral valve interventions with minimal or no septal resection for adequate relief.
Advertisement
The underlying mechanisms involve specific mitral valve leaflet and subvalvular apparatus abnormalities that allow flow vortices to induce SAM and LVOTO, often with significant MR. These abnormalities include:
“Understanding these mechanisms is critical for guiding surgical strategies that go beyond traditional septal myectomy alone,” Dr. Smedira observes.
The authors present their toolkit of tailored surgical techniques for mitral valve repair and replacement, developed and refined over 30 years. Mitral valve repair is favored when possible, with the approach in this setting founded on two key principles: (1) modifying the abnormal effects of flow vortices and (2) restoring a more posterior leaflet coaptation zone.
“The selection of specific techniques is guided by careful preoperative imaging — including echocardiography and cardiac MRI — to assess leaflet elongation, papillary muscle mobility, hypertrophy and anterior displacement, along with LVOT gradients at rest and under provocation,” says co-author Milind Desai, MD, MBA, Medical Director of the Hypertrophic Cardiomyopathy Center. Following this initial preoperative evaluation, definitive assessment takes place intraoperatively, via direct visualization.
Advertisement
The toolkit for mitral valve repair, often performed through a transaortic approach, includes the following:
Advertisement
While repair is always the favored option, mitral valve replacement is sometimes necessary, especially in patients with significant mitral annular calcification or with thickened/calcified leaflets that restrict motion and prevent adequate repair.
The Cleveland Clinic authors often implant stent-mounted bioprosthetic valves. They recommend use of a transverse aortotomy to place the valve suture in the very middle of the LVOT under direct visualization, ensuring careful strut orientation to achieve a wide-open LVOT and prevent strut-induced obstruction. They also underscore the importance of differentiating between true and false annuli to avoid ventricularization of the prosthesis and subsequent LVOTO.
“Our 30-year experience demonstrates that mitral surgery can effectively eliminate SAM, relieve LVOTO and resolve MR in patients with mitral valve-induced obstruction and minimal or no septal hypertrophy,” says Dr. Smedira. “Our toolkit’s tailored, pathoanatomically guided techniques often allow for durable repair. When repair is not possible, bioprosthetic mitral valve replacement offers a safe alternative, crucially sparing patients, especially younger ones, from lifelong anticoagulation.”
He and his co-authors note that their use of mitral-based strategies in these cases usually does not exclude myectomy entirely. “In our practice,” they write, “approximately 85% of patients receive a concomitant limited septal myectomy weighing 3 g or less to comprehensively address all possible mechanisms of LVOTO.” The key, they note, is to limit the myectomy to what’s truly necessary, as excessive septal resection in the setting of minimal hypertrophy raises the risk of ventricular septal defect.
Advertisement
Moreover, the clear anatomical contribution of the mitral valve to LVOTO in these cases strongly supports the utility of mitral intervention. “Even when a limited myectomy is performed, it may not fully relieve obstruction, making these adjunctive mitral techniques invaluable,” Dr. Desai points out. “Recognizing these distinct mechanisms of LVOTO and the efficacy of mitral-focused surgical strategies is paramount for accurate diagnosis, appropriate referral and optimal understanding of potential postoperative outcomes.”
Advertisement
Modified-Bentall single-patch Konno enlargement (BeSPoKE) optimizes hemodynamics, facilitates future TAVR
Scenarios where experience-based management nuance can matter most
NIH-funded comparative trial will complete enrollment soon
Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic
Why and how Cleveland Clinic achieves repair in 99% of patients
End-of-treatment VALOR-HCM analyses reassure on use in women, suggest disease-modifying potential
Two surgeons share insights on weighing considerations across the lifespan
Cardiac imaging substudy is the latest paper originating from the VANISH trial