Hyperthermia, neoadjuvant chemoradiation and reconstruction hold promise of long-term survival
A 66-year-old woman treated for invasive ductal carcinoma with lumpectomy, lymph node biopsy, and MammoSite-accelerated partial breast irradiation a decade before presented to Cleveland Clinic with a 1-year history of burning discomfort in the left upper quadrant of her abdomen and chest.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Based on a limited workup, the patient was treated for presumed gastroesophageal reflux disease with a proton pump inhibitor, with no relief. She then noticed palpable changes at the site of the prior lumpectomy, which were initially attributed to radiation fibrosis. A routine screening mammogram led to the discovery of an 8-cm mass in the lower outer aspect of the patient’s left breast, which had not been present on a mammogram done 16 months before. A core needle biopsy of the mass showed that it was a large cell malignant neoplasm; immunostaining was positive for undifferentiated pleomorphic sarcoma but a sarcomatoid carcinoma could not be ruled out.
On magnetic resonance imaging (MRI), the mass measured 14.0 x 9.0 x 9.0 cm and was seen to be invading the chest wall. Involvement of the pleura, pericardium, and left hemi-diaphragm also was suspected. No evidence of infiltration was found on a follow-up MRI of the brachial plexus.
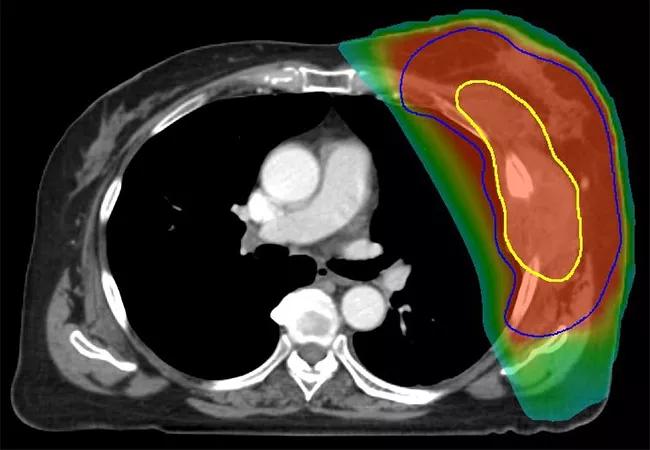
Following evaluation by a multidisciplinary team at Cleveland Clinic, the patient received neoadjuvant chemoradiation with 50 Gy in 25 fractions delivered once daily, with a 1-cm bolus applied to the chest wall for the first 13 fractions. Superficial hyperthermia was administered with a 915 MHz microwave applicator twice a week, separated by 72 hours, with a target temperature of 40°C 60 for 1 hour immediately prior to radiation. The patient also received gemcitabine concurrently at a dose of 500 mg/m2 weekly.
Five weeks after the patient completed chemoradiation, a contrast-enhanced computed tomography scan of the chest showed no significant change in the size of the primary mass. Expected post-radiation changes, including asymptomatic radiation pneumonitis and a small pleural effusion, were visible. Given the lack of evidence of distant metastasis, surgical resection was performed 2 weeks later. Because the patient’s disease was locally advanced, the procedure included resection of the chest wall, portions of ribs 3 to 8, and a wedge resection of the lung lingula. Titanium mesh measuring 20 x 20 cm was used for reconstruction of the 18-cm postoperative defect; prolene mesh was used to repair a small residual defect. Following wound closure, a Cleveland Clinic plastic surgery team used a latissimus dorsi myocutaneous flap, pectoralis minor flap advancement, pectoralis major muscle flap advancement, and serratus muscle flap advancement to perform the multisite reconstruction.
Advertisement
Pathology showed that the patient’s mass was a ypT3 primary tumor with 70% necrosis; the final margins were negative. She tolerated surgery well, was discharged 5 days after the procedure, and had no evidence of disease or disability 21 months post-resection.
Soft-tissue sarcoma (STS) is a known complication of breast irradiation, and outcomes in patients with the disease are usually poor. In this case, because ABPI was delivered with an older MammoSite device, the radiation distribution was not well-optimized to limit the dose to the chest wall and skin. The patient’s tumor was so large that it was initially believed to be unresectable. The use of neoadjuvant therapy was crucial because it enabled resection with limited margins and afforded the opportunity to assess the biologic behavior of the tumor. Hyperthermia was used because it enhances perfusion, which improves oxygenation and possibly the effectiveness of chemoradiation.
Few studies have been published on use of neoadjuvant thermochemoradiation followed by a large surgical resection that requires extensive reconstruction. “Our case suggests that an aggressive, multimodal approach may be appropriate for carefully selected patients who have STS of the chest wall,” says Shauna Campbell, DO, of the Department of Radiation Oncology at Cleveland Clinic. “The potential for long-term survival exists for patients with this challenging disease who do not have metastasis if they have the benefit of evaluation by a multidisciplinary team at a tertiary care center.”
Advertisement
Advertisement
Advertisement

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units

Addressing rare disease and challenging treatment course in an active young patient