Minimizing the translational gap between preclinical testing and clinical outcomes
Human coronaviruses (HCoV), including Coronavirus Disease 2019 (COVID-19), can lead to epidemics with high morbidity and mortality. These epidemics emerge and mutate at such a rapid rate that traditional methods of drug discovery cannot keep up.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The challenge de novo drug discovery is that it takes a long time – say 15 years or longer — to get a drug from discovery through FDA approval for clinical use. This traditional discovery method cannot be used to efficiently or effectively find treatments for epidemics like COVID-19,” says Feixiong Cheng, PhD, a researcher with the Cleveland Clinic Lerner Research Institute.
There are currently no effective drugs approved to treat COVID-19. However, since viruses depend on host proteins to replicate and spread, existing drugs that target those proteins could prove to be effective antiviral therapies. Drug repurposing, or the repositioning of existing drugs for new therapeutic purposes, could be an efficient and cost-effective approach to establishing prevention and treatment strategies for COVID-19. In a paper published today in Cell Discovery, Dr. Cheng applies his network-based prediction model to identify targets for drug repurposing in HCoV, and COVID-19 specifically.
Traditional antiviral discovery generally targets the virus protein; however, the virus genome evolves quickly. “Our approach targets the interaction between human and virus proteins rather than the virus protein itself,” Dr. Cheng says.
Dr. Cheng’s team obtained the host proteins that are either directly targeted by HCoV or are involved in crucial pathways of HCoV infection and compared them to known drug-target interactions. Based on their findings, they prioritized 16 drugs and three drug combinations as potential treatments for HCoVs, including COVID-19. Importantly, the authors discussed that network-identified repurposable drugs offer ideal candidates for adjuvant therapeutic agents by combining with existing antiviral drugs (e.g., remdesivir).
Advertisement
“We computationally identified drugs that may be repurposed for use in newer, evolving diseases like coronavirus. The beauty of drug repurposing is that we are looking at drugs that are already approved by the FDA, so we know their pharmacokinetic properties and toxicity profiles,” Dr. Cheng states.
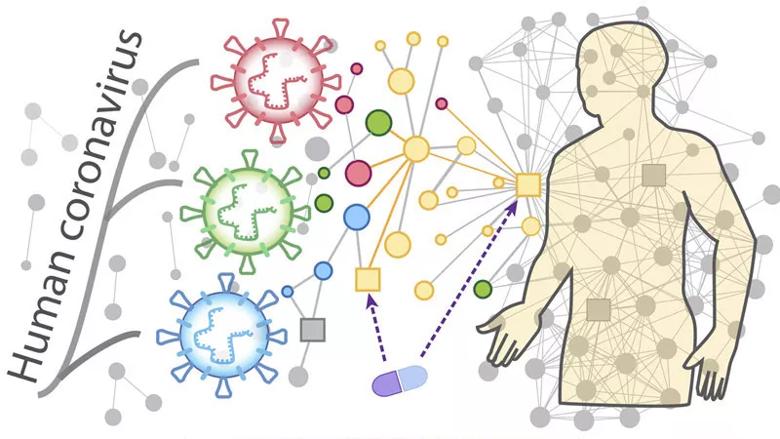
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2d8f1da5-400c-4c35-b134-c58190731855/Good20-LRI-1855227-drugRepurposeCoronovirus-inset-800x450-1_jpg)
Overall hypothesis of the network-based methodology: (i) the proteins that functionally associate with HCoVs are localized in the corresponding subnetwork within the comprehensive human interactome network; and (ii) proteins that serve as drug targets for a specific disease may also be suitable drug targets for potential antiviral infection owing to common protein–protein interactions elucidated by the human interactome.
Drug combinations may increase clinical efficiency and reduce toxicity for patients with COVID-19, as previously demonstrated in various other antiviral therapies, such as human immunodeficiency virus (HIV) and H1N1 flu virus. In this recent study, Dr. Cheng discussed three potential drug combinations that may prove effective in the treatment of COVID-19. These include:
Advertisement
Preclinical and clinical studies are necessary to validate these potential treatments. However, this study offers an approach that can minimize the translational gap between preclinical testing and clinical outcomes, which is a significant barrier to the rapid development of treatment strategies for the emerging COVID-19 outbreak.
Dr. Cheng hopes to collaborate with other health systems and national/international investigators to test the network-predicted repurposable drugs and drug combinations in preclinical models and clinical applications within the next couple of months. Finally, Dr. Cheng highlights that big data sharing and research collaboration are essential to fight the rapid COVID-19 outbreak.
Image note: The rendering of COVID-19 is courtesy of the Centers for Disease Control and Prevention.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology