Evidence-based recommendations for balancing cancer control with quality of life
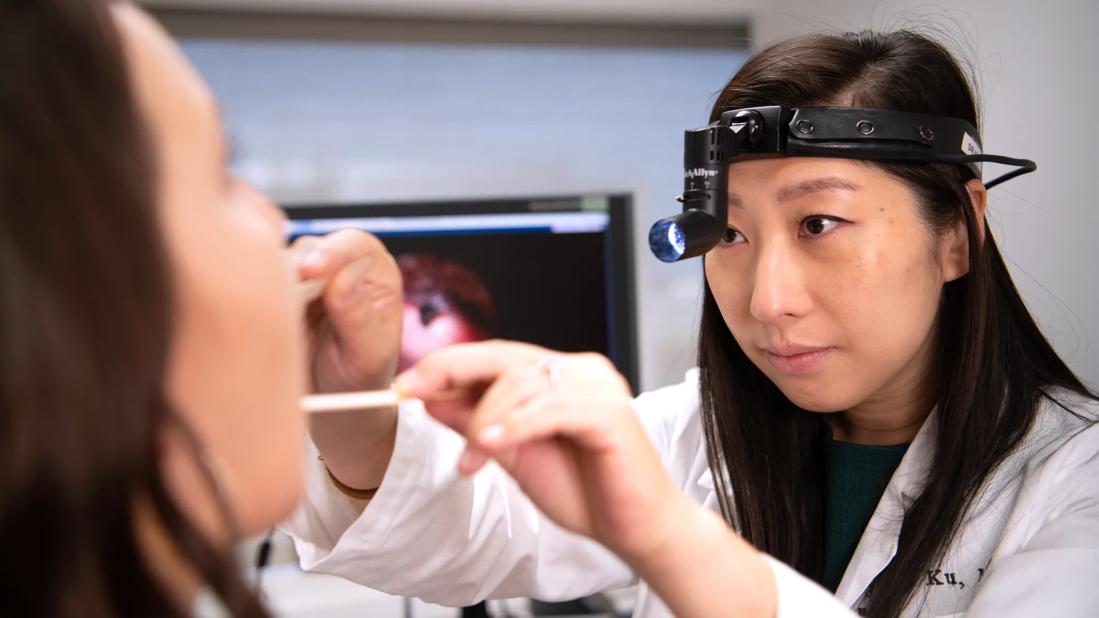
As rates of oropharyngeal squamous cell carcinoma (OPC) continue to rise — particularly in cases linked to human papillomavirus (HPV) — clinicians are increasingly tasked with balancing robust cancer control outcomes with long-term quality of life. A newly published American Society of Clinical Oncology (ASCO) guideline, “Transoral Robotic Surgery in the Multidisciplinary Care of Patients With Oropharyngeal Squamous Cell Carcinoma,” aims to address that challenge by providing evidence-based recommendations on the use of transoral robotic surgery (TORS) in the multidisciplinary care of patients with OPC.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Traditionally, OPC has been treated nonsurgically with chemoradiation — usually 70 Gy of radiation combined with cisplatin-based chemotherapy. This approach is highly effective and has a 5-year overall survival rate of 85%. However, it is also associated with significant acute and late toxicities, including dysphagia or swallowing dysfunction.
Over the past decade, multiple prospective trials have explored strategies to de-escalate this intense treatment regimen, first by substituting cisplatin with cetuximab, and later by lowering the radiation dose. Yet, none have successfully demonstrated comparable outcomes to the standard 70 Gy with cisplatin. As a result, there is a continued effort to find alternative, less toxic approaches. One such alternative is TORS, which may allow for comparable oncologic control with potentially fewer long-term side effects in appropriately selected patients or allow for less radiation or even omission of chemotherapy.
“Transoral robotic surgery has emerged as a promising option for many patients. However, until now, there wasn’t a strong consensus or evidence-based guideline to help surgeons or multidisciplinary teams determine which patients are the best candidates for TORS and which may not be,” says Jamie Ku, MD, an otolaryngology-head and neck cancer surgeon in Cleveland Clinic’s Head and Neck Institute and the Co-Chair and the senior author of the ASCO guideline publication. “We saw an opportunity to create a more surgically focused guideline and one that could evaluate the existing evidence and provide thoughtful recommendations around surgical options.”
Advertisement
Given the growing interest in transoral robotic surgery as a tool for potential treatment de-escalation in oropharyngeal cancer, the ASCO Expert Panel sought to answer a key clinical question: How and when should TORS be used in the multidisciplinary setting for primary and recurrent cancers of the oropharynx?
To address this, the panel focused the guideline specifically on traditional TORS cases — namely, T1 and T2 tumors without the need for reconstruction. While TORS can be used in a variety of clinical scenarios, including more advanced disease and other histologies, this guideline intended to provide clear, evidence-based recommendations for preoperative assessment, patient selection, and multidisciplinary adjuvant treatment planning for oropharyngeal cancer patients.
“We assembled a multidisciplinary panel of experts to review the literature and develop consensus recommendations with strength ratings for both the recommendations and the quality of evidence,” notes Dr. Ku. “The final product is a comprehensive, evidence-informed guideline.”
The ASCO Expert Panel was composed of clinicians across specialties, a patient representative and a health research methodologist. The group conducted a systematic literature review covering studies published from January 1, 2002, to August 31, 2024. A total of 58 publications met the inclusion criteria, comprising of systematic reviews, meta-analyses, randomized controlled trials, and observational studies. Eligible studies focused on patients with OPC and reported outcomes such as overall survival (OS), disease-free survival (DFS), functional outcomes, and quality of life.
Advertisement
Studies were excluded if they were unpublished meeting abstracts, narrative reviews, case reports, non-English publications, or editorials. Each recommendation in the guideline is accompanied by an evidence quality rating, assessed using the Cochrane Risk of Bias tool and the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach.
Throughout the development process, the panel held four full meetings and collaborated in five working subgroups to ensure that recommendations were comprehensive and multidisciplinary. Draft recommendations were opened to public comment in June 2024, with 89% of responses in agreement or agreement with minor revisions. Only 11% expressed disagreement. Comments were carefully reviewed and incorporated before final approval by both the panel and ASCO’s Evidence-Based Medicine Committee.
The newly released ASCO guideline offers timely, focused guidance on how to incorporate TORS into the care of patients with OPC, particularly in a multidisciplinary setting. Among the most significant contributions of the guideline are clear recommendations on patient selection, preoperative evaluation, the role of adjuvant therapy, and considerations for HPV-positive and HPV-negative disease. It also explores the use of TORS in salvage or recurrent cases.
An important focus of the guideline is appropriate patient selection—a key factor in optimizing outcomes and minimizing harm. According to the expert panel, TORS should be discussed as a treatment option for patients with T1-T2 HPV-positive OPC, particularly when preoperative imaging suggests a high likelihood of achieving an R0 resection. While TORS is FDA-approved for T1 and T2 tumors, the authors note it may be considered for select exophytic T3 tumors if resection is feasible without major functional compromise. T4 tumors, however, are not suitable for transoral resection.
Advertisement
Patients with lateralized tumors are considered ideal candidates. The panel also stresses the importance of evaluating anatomic and functional suitability, including factors such as jaw structure, trismus, tongue base access, and neck mobility. Patients with features limiting exposure, such as a narrow mandibular arch or poor neck extension, may not be appropriate candidates.
“Patient selection is critical,” emphasizes Dr. Ku. “We wanted to ensure that TORS is being applied to the right patients—those who are most likely to benefit—while also recognizing that the level of evidence varies across clinical scenarios.”
Another cornerstone of the guideline is the emphasis on comprehensive, multidisciplinary evaluation. The panel underscores that all patients being considered for TORS should be evaluated upfront by a team that includes not only cancer-treating clinicians, like radiation oncologists, medical oncologists, and head and neck surgeons, but also supportive care specialists. This includes speech-language pathologists and rehabilitation therapists, who play a vital role in pre-treatment swallowing assessments and long-term quality of life planning.
“These are complex conversations, and patients need to be actively involved,” adds Dr. Ku. “The inclusion of specialists like speech-language pathologists enriches the shared decision-making process.”
At Cleveland Clinic, the guideline largely validates existing practices. The head and neck cancer team already operates with a strong multidisciplinary model and routinely integrates surgical and nonsurgical options based on each patient’s specific needs.
Advertisement
“We don’t anticipate a major shift in how we deliver care,” Dr. Ku shares. “But what we do hope is that the guideline helps expand this standard of care beyond the main campus to regional and community providers and frontline clinicians who may be the first to encounter these patients.”
That includes primary care providers, oral surgeons, dentists, general otolaryngologists, and others who may be well-positioned to identify potential surgical candidates early in the care pathway.
Looking ahead, the guideline also identifies key areas for future research, particularly where the evidence base is limited or evolving. Topics such as circulating tumor DNA, immunotherapy integration, the prognostic importance of extranodal extension, and treatment personalization based on molecular profiling are likely to shape the next wave of innovation in OPC management.
“There are still questions we need to answer, and some areas where the data just isn’t strong enough yet,” Dr. Ku concludes. “But the direction we’re heading in is exciting.”
Advertisement

Screening also found to be cost-effective

Looking at short-term outcomes in a high-risk population

Research aims to better understand the tumor immune micorenvironment
Prompt surgery was necessary when symptoms drastically increased
A new single-port system well-suited for oropharyngeal cancer treatment

Use of MRI adds to cost but remains cost-effective due to higher sensitivity

Analysis of HNSCC patients shows HPV status to be predictive of higher abundance of oncobacteria within the tumor

Case study illustrates the potential of a dual-subspecialist approach