Refractory patients respond to combination therapy
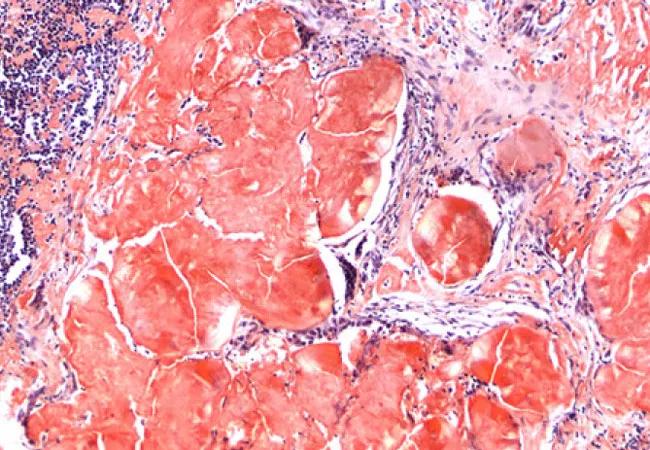
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c78fdb02-8648-418b-b2cb-b192151b0cda/amyloid_650x450_jpg)
amyloid_650x450
Light chain or primary amyloidosis (AL) is a rare disease that can cause serious comorbidities. Amyloid protein buildup in major organs, such as the heart, liver and kidneys, impairs function and in severe cases can lead to organ failure. These comorbidities can make tolerating medications difficult, especially in patients with congestive heart failure (CHF).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Since it originates in the bone marrow, amyloidosis responds to the same chemotherapy drugs as multiple myeloma (MM). At an early stage, the treatment goal is to completely stop the growth of abnormal cells that produce amyloid and improve organ function which can allow patients to have a normal lifespan.
For the past six years, the first-line medical treatment has been combination therapy with cyclophosphamide, bortezomib and dexamethasone (CyBorD). However, most patients do not achieve a complete response (CR) with this treatment. A newly approved drug, daratumumab (DARA), a CD38-directed monoclonal antibody, has revolutionized MM care and has been shown to be safe and highly effective in treating relapsed/refractory AL amyloidosis.
Cleveland Clinic Cancer Center investigated therapy with DARA, bortezomib and dexamethasone in AL patients who did not achieve at least a very good partial response (VGPR) to initial treatment with CyBorD. “This regimen has proved effective in treating multiple myeloma with three drugs being more effective than two,” says Jason Valent, MD, an oncologist in the Department of Hematology and Medical Oncology.
Since the approval of DARA for MM, Cleveland Clinic has seen 159 patients with newly diagnosed AL, of which 10 failed to achieve hematologic VGPR with CyBorD treatment. The cohort was chosen from the clinic’s IRB-approved plasma cell disorder registry. The median age was 67, and 80 percent were males.
At the time of diagnosis, 50 percent of the patients had more than one organ involvement, which included heart, kidney, lung, gastrointestinal system and/or bone marrow. All patients received initial treatment with CyBorD (an average of four cycles) with the following results: 60 percent had a partial response, and 40 percent had stable disease. The majority of patients (70 percent) had a second-line regimen of DARA, bortezomib and dexamethasone; the remainder had DARA monotherapy. The average number of cycles was five, with 50 percent of patients undergoing treatment at the time of analysis.
Advertisement
The overall hematologic response rate was 90 percent. Five patients achieved at least a VGPR, and two patients achieved a CR. By an average of two cycles, 70 percent of patients had improved organ function. Cleveland Clinic policy is to eliminate dexamethasone as a premedication after three DARA doses to minimize the risk of volume overload. No hospitalizations or deaths attributed to therapy occurred.
“The DARA regimen was very well-tolerated, and we saw a response in just three weeks, which is stunning,” says Dr. Valent. “When you shut down light chain production, protein buildup quickly decreases. Patients who were very symptomatic regained organ function, which is critical to prevent irreversible organ damage.”
Study results were presented at the American Society of Hematology meeting in December.
Clinic investigators are planning further study of DARA as a first-line treatment. “We are interested in finding out whether CyBorD is needed at all when DARA is used in newly diagnosed patients,” says Sarah S. Lee, MD, a study investigator and fellow in hematology/oncology.
Image: Pathology examination of the right cervical lymph node (magnification × 100). Amyloid with Congo-red positivity. Reused with permission from a case study Dr. Valent published in Chest.
Advertisement
Advertisement
Clinical trials and de-escalation strategies
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality