Encouraging results for an incurable bone cancer
A multicenter phase 1 trial of ibrunitib, carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (RRMM) demonstrated a 67 percent overall response rate. Jason Valent, MD, associate staff in the Department of Hematology and Medical Oncology at Cleveland Clinic Cancer Center, is enthusiastic about the results of the study in which Cleveland Clinic was a primary participant.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Multiple myeloma is an incurable bone cancer, normally managed sequentially with different drug therapies. About 90 percent of patients initially respond to standard backbone therapies, which include carfilzomib, bortezomib, lenalidomide and/or pomalidomide in combination with dexamethasone. However, relapse is inevitable for almost all patients. For patients refractory to chemotherapy, expected survival is only nine months.
In the search to extend life expectancy and improve quality of life for RRMM patients, authors of this prospective study, published in Leukemia and Lymphoma, tested the efficacy of an ibrunitib-carfilzomib-dexamethasone combination. They also sought to determine the highest safe dosage for ibrunitib (840 mg) for use in the phase 2 trial, currently underway.
“In relapsed disease, generally three drugs are better than two drugs. In this case, the backbone of carfilzomib and dexamethasone was expanded on with the addition of ibrutinib,” Dr. Valent explains.
Forty-three patients from 13 centers were enrolled. All had received two or more lines of therapy previously, including bortezomib and an immunomodulator. Nearly a third of the patients discontinued treatment because of adverse events, a lower number than expected in this very ill population.
Treatment showed efficacy for 76 percent of the remaining patients. This compared very favorably with the 19-27 percent efficacy rate in patients who take carfilzomib and dexamethasone alone. Two percent of the patients demonstrated a “stringent complete response,” 21 percent had a “very good partial response,” 43 percent achieved a “partial response” and 10 percent had a “minimal response.” Median progression-free survival was 7.2 months, compared with a four-month expectancy rate in trials with similar patient populations.
Advertisement
Dr. Valent explains that patients this sick will typically suffer a broad range of side effects from chemotherapy, with cardiac toxicity one of the chief concerns. Ibrutinib is known to put patients at some risk for atrial fibrillation, with carfilzomib potentially causing respiratory or heart failure in about 7 percent of patients. Yet, in this study, the combination did not appear to increase the incidence of these side effects.
Ibrunitib, a tyrosine kinase inhibitor, targets the expression of myeloma cells’ BTK receptors, which are involved in promoting the division and growth of multiple myeloma cancer cells. It works by docking at the receptor site, blocking BTK stimulation and thereby disrupting the signal pathway the cell depends on to proliferate. Ibrunitib is known to be particularly effective in hematologic cancers; it is FDA approved for chronic lymphocytic leukemia and certain types of lymphoma. Using it to attack multiple myeloma, however, represents a novel application.
“Interestingly, one of the Cleveland Clinic patients in the trial who had not responded well to initial three lines of treatment had an exceptional response to the ibrunitib-carfilzomib-dexamethasone combination,” Dr. Valent recounts. “He tolerated the drug very well and is in his fourth year of progression-free remission — and counting.” If the BKT receptor theory is accurate, it may be that certain patients, like this one, have a particularly high level of BTK expression, explaining the strong positive response to this treatment.
Advertisement
“This study showcases a durable, safe and pretty spectacularly effective combination,” Dr. Valent says. “When patients are tripling their response rate and nearly doubling their progression-free survival rate without increased side effects, we are onto something important.”
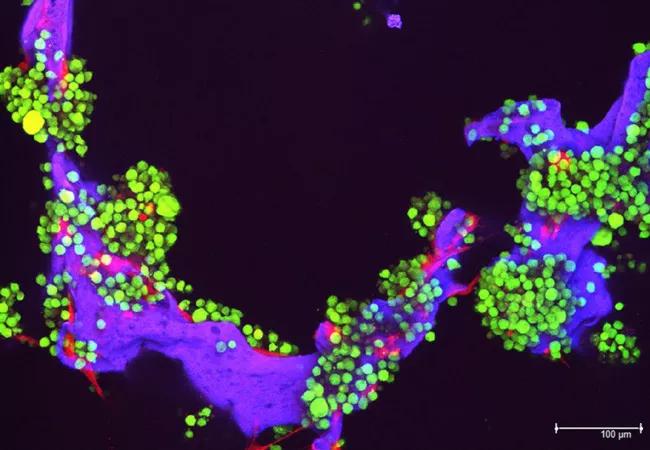
Image: Myeloma tumor cells (in green) and bone cells (red) growing on a scaffold made of silk protein (purple), which is designed to resemble bone material. Source: National Cancer Institute Visuals Online.
Advertisement
Advertisement

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units

Addressing rare disease and challenging treatment course in an active young patient