Study combines intracranial electrophysiology and SPECT to elucidate the role of hypoperfusion
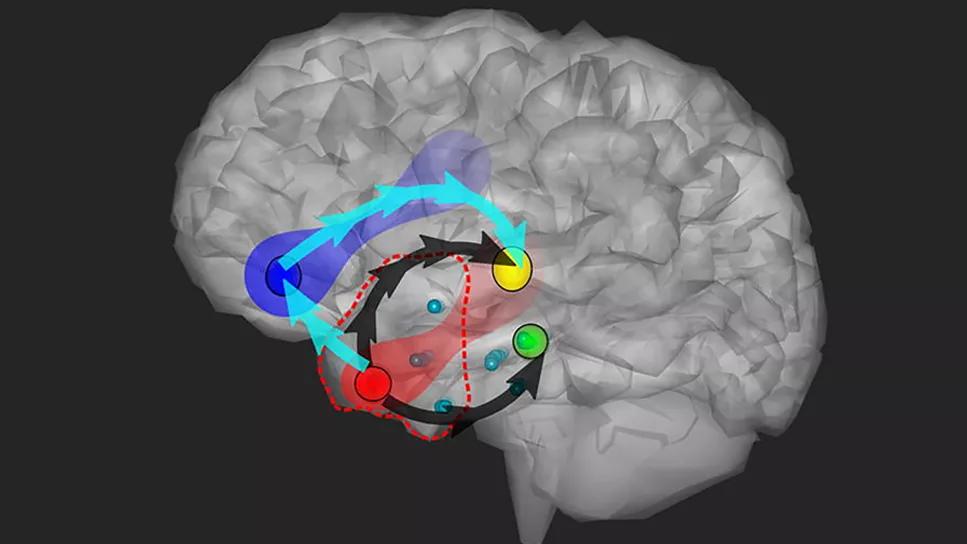
Cleveland Clinic researchers have characterized the intricate dynamics of information flow within epileptic networks during the transition from interictal to ictal states. Their findings, from a study published in Clinical Neurophysiology (2024;161:80-92), demonstrate that hypoperfused brain regions interact indirectly with the epileptogenic zone during the ictal period and could serve as nodes of a potential control network for therapeutic targeting.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
By combining intracranial stereoelectroencephalography (SEEG) and ictal SPECT data, the study offers new and potentially useful insights into the complex interactions among brain regions exhibiting distinct perfusion patterns during seizures.
“It is recognized that hyperperfused brain regions are frequently associated with seizure onset and propagation, but the significance of hypoperfusion during seizures has been uncertain,” says the study’s corresponding author, Balu Krishnan, PhD, a research scientist in Cleveland Clinic’s Epilepsy Center. “We undertook this investigation using multimodal, quantitative methodology to systematically describe the spatio-temporal information flow dynamics between brain regions with different levels of perfusion.”
Working with research and clinical colleagues in Cleveland Clinic’s Epilepsy Center, Dr. Krishnan retrospectively analyzed consecutive patients with drug-resistant focal epilepsies who underwent surgical resection at Cleveland Clinic over a two-year period. For inclusion, patients had to have undergone both an ictal SPECT assessment and a subsequent presurgical SEEG evaluation showing at least one seizure. Patients also needed to be seizure-free following resection and to have a high-quality postoperative MRI.
Overall, 10 patients (median age at surgery, 26 years) met these inclusion criteria and had a total of 24 seizures that could be analyzed.
Using the technique known as directed transfer function, the researchers evaluated preictal, ictal and postictal connectivity across the 24 seizures, with a specific focus on interaction among brain regions with differing perfusion patterns. Their key findings included the following (also see Figure below):
Advertisement
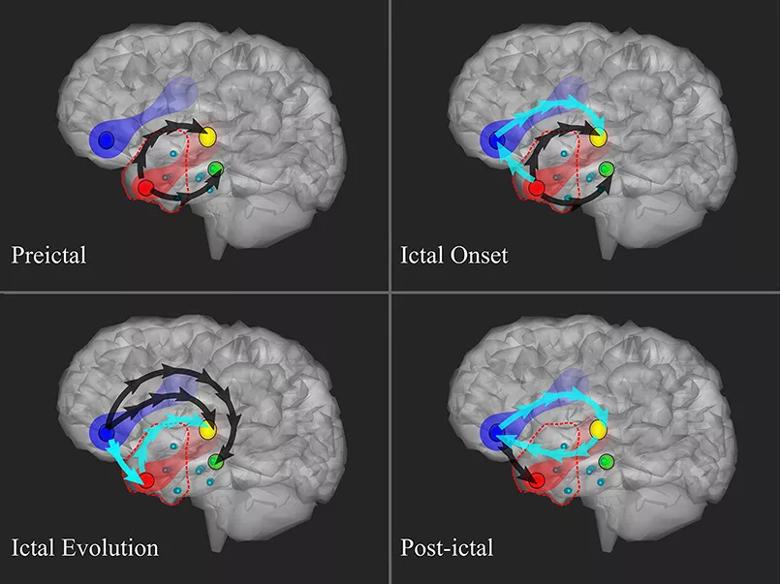
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/de837732-e224-4b9e-92c8-15e48db8ea51/brain-connections-during-seizure)
Figure. Schematic summary of directional connectivity between the epileptogenic zone (red node) and the hyperperfused (yellow node), hypoperfused (blue node) and baseline-perfused (green node) brain regions during the stages of a seizure. Arrows indicate the existence of a statistically significant increase in connectivity with respect to the background; black arrows denote increased low- and high-frequency information flow, while cyan arrows denote increased low-frequency connectivity. Reproduced from Krishnan et al., Clinical Neurophysiology (2024;161:80-92), ©2024 International Federation of Clinical Neurophysiology. Published by Elsevier BV. Used under a Creative Commons license (CC BY-NC-ND 4.0).
The researchers note that their findings have significant implications for clinical practice and future epilepsy research, including the following:
“Additionally, these findings highlight the importance of multimodal integration of electrophysiological and imaging data to enhance the diagnosis and treatment of drug-resistant focal epilepsies,” Dr. Krishnan says. “This type of approach promises to provide complementary diagnostic information and aid in the accurate delineation of patient-specific epileptogenic networks.”
The researchers acknowledge several limitations to their study, including a small patient population limited to temporal and frontal lobe epilepsies, as well as the absence of electrophysiological recordings from subcortical regions. Also, the prevalence of focal cortical dysplasia in the study population may have influenced perfusion characteristics.
Advertisement
“Nevertheless, these data shed light on the complex dynamics of epileptic networks, offering a novel perspective on the role of ictal hypoperfusion in seizure propagation and evolution,” Dr. Krishnan observes. “They set the stage for future investigations that could lead to improved surgical strategies, targeted neurostimulation approaches and development of seizure prediction algorithms, ultimately enhancing the clinical management of patients with drug-resistant focal epilepsies.”
He adds that future research should aim to validate these findings across a larger and more varied patient population, incorporating various histopathologies and epilepsy types. Additionally, well-designed studies combining intracranial SEEG with functional neuroimaging techniques, such as EEG-fMRI, could further elucidate the role of subcortical structures in the interactions between hypoperfused brain regions.
Images are reproduced from Krishnan et al., Clinical Neurophysiology (2024;161:80-92), ©2024 International Federation of Clinical Neurophysiology. Published by Elsevier BV. Used under a Creative Commons license (CC BY-NC-ND 4.0).
Advertisement
Advertisement

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Patients with epilepsy should be screened for sleep issues

Sustained remission of seizures and neurocognitive dysfunction subsequently maintained with cannabidiol monotherapy
Model relies on analysis of peri-ictal scalp EEG data, promising wide applicability

Investigational gene approaches offer hope for a therapeutically challenging condition

Characterizing genetic architecture of clinical subtypes may accelerate targeted therapy

Data-driven methods may improve seizure localization and refine surgical decision-making

Pre-retirement reflections from a pioneering clinician, researcher and educator