TRAF4 may be a viable therapeutic target
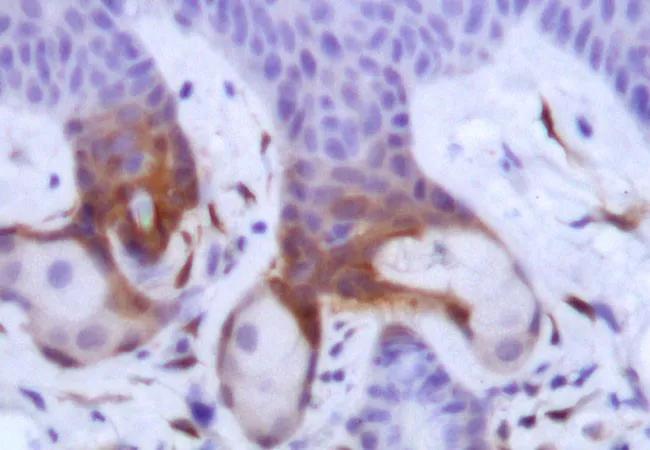
Previous research has shown that uncontrolled tissue repair is associated with tumor formation (tumorigenesis), although the direct connection between the two was not previously been well defined.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Lerner Research Institute scientists, led by Xiaoxia Li, PhD, Department of Inflammation & Immunity, discovered a new signaling pathway in a subset of hair follicle stem cells that mechanistically links for the first time inflammation, wound healing and tumorigenesis, offering new insights into this disease-driving process and suggesting a potential target to slow or prevent tumor initiation.
The research team found in a preclinical model that the proinflammatory cytokine IL-17A (interleukin-17A)—of which high levels are associated with several chronic inflammatory diseases and cancer—plays a critical role in aberrant tissue repair. When IL-17A signaling was “turned on,” Lrig1+ stem cells, found in the skin’s hair follicles, multiplied and their progeny translocated to other layers of the skin in response to injury and there played an active role in epidermal tumorigenesis.
Looking to take their investigation a step further, the researchers set out to understand what happens between activation of the IL-17A signaling cascade and tumorigenesis, as they knew the “in between” is where an actionable therapeutic target is likely to live.
Through a series of signaling assays, Dr. Li and collaborators showed that the presence and physical proximity of a series of proteins sets into motion a complex signaling cascade that results in activation of ERK5 (extracellular signal regulated kinase 5), which is ultimately responsible for the expansion and migration of Lrig1+ stem cells. While many proteins—including IL-17R (interleukin-17 receptor), EGFR (epidermal growth factor receptor) and Act1—are involved, TRAF4 (tumor necrosis factor receptor associated factor 4) is the first domino to fall, setting the entire signaling cascade in motion.
Advertisement
“This study is important for two reasons. On one hand, it is the first study to show that a proinflammatory cytokine can recruit a growth factor receptor to activate stem cells in support of tissue repair and tumorigenesis,” explains Dr. Li. “It also identified TRAF4’s upstream role in tumor development.”
Considered together with the fact that others in the field have found the protein to also aid in tumor growth, the team believes TRAF4 may be a viable therapeutic target to pursue in future studies.
Xing Chen, PhD, is first author on the study, which was published in the Journal of Experimental Medicine, and supported by grants from the National Cancer Institute and National Heart, Lung, and Blood Institute, both parts of the National Institutes of Health. Dr. Li holds the Paul L. Fox, PhD, Endowed Chair in Molecular Medicine at Cleveland Clinic’s Lerner Research Institute. Drs. Brian Gastman, Jennifer Ko, Shlomo Koyfman and Allison Vidimos provided insight into the clinical relevance of this study.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology