Retroperitoneal approach now possible for anteriorly located kidney tumors
A new single-port robotic surgical system, which recently received U.S. Food and Drug Administration clearance, has potential to overcome a number of restrictions encountered with existing platforms. The technology makes robotic laparoendoscopic single-site (R-LESS) surgery possible through a single incision about 1 inch long.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Usually, the location of a tumor in the kidney dictates whether or not we can use a retroperitoneal approach,” explains Jihad Kaouk, MD, Director, Center for Robotic and Image-Guided Surgery, Glickman Urological & Kidney Institute. “This approach can be advantageous because it allows us to avoid going through the abdomen and the need to displace the bowel. With current robotic technology, only kidney tumors located posteriorly or laterally are amenable to the retroperitoneal approach. If it is anterior, you cannot see it.”
This new robotic system changes that. It makes a retroperitoneal approach possible even for anteriorly located kidney tumors.
A study by Dr. Kaouk and others published in the European Journal of Urology demonstrated successful deployment of the system in five cadavers. One retroperitoneal radical nephrectomy and four retroperitoneal bilateral partial nephrectomies were performed on both anterior and posterior surfaces of the kidney.
All procedures were completed without complication and without the need for conversion to conventional surgery.
“We successfully performed major surgery on the kidney through a single small incision without entering the abdomen,” notes Dr. Kaouk.
Since the preclinical study was published, and following Institutional Review Board approval, Dr. Kaouk led a team that performed the initial clinical cases of partial nephrectomy in collaboration with Université Lille Nord de France, Lille, France. The procedures, performed in 2010, were successful and published in the European Journal of Urology.
Advertisement
Dr. Kaouk has worked for several years in close collaboration with the manufacturer to develop and test this new purpose-built R-LESS system.
“It offers notably better dexterity for the surgeon and less morbidity compare to current robotic technologies,” he continues. Major improvements include delivery of three double-jointed articulating instruments through a single port into the surgical field, in addition to an articulating 3D high-definition camera, plus a laparoscopic accessory instrument.
Additional improvements include automated monitoring of instruments’ position within the surgical field and alerts that notify the surgeon when instruments are nearing the limits of their range.
The device has shown proof of concept for robotic laparoscopic interventions in several other procedures in addition to partical nephrectomy. These include pyeloplasty and radical prostatectomy, Dr. Kaouk notes.
An article published in the British Journal of Urology shows proof of preclinical concept for cystoprostatectomy and bilateral extended pelvic lymph node dissection (ePLND) by conducting the surgery on three male cadavers.
No conversions to conventional surgery were required and no intraoperative complications were observed. The median operating time for radical perineal prostatectomy and pelvic lymph node dissection was 210 minutes with procedures ranging in duration from 180-240 minutes.
“Use of this single-port system could allow surgeons to perform major urological and other operations via a single, small incision while preserving triangulation and optics, and eliminating clashing between instruments.”
Advertisement
Surgeons will need to invest practice time on devices before attempting to use the technology in patients.
“Techniques like these stand to offer improvements in quality of life for our patients, including reduced recovery times and less scaring,” Dr. Kaouk comments, adding that with measured and careful implementation, the system “will be just the beginning of a new generation of surgical robots.”
Dr. Kaouk has no investments in the device manufacturer or the device itself.
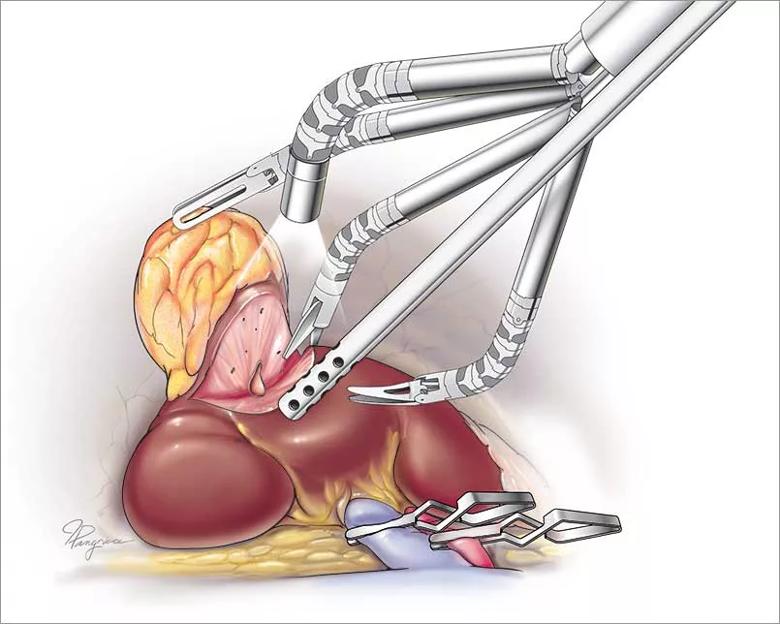
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1cb6b146-ca24-4592-90ab-57739f32cfc9/805x-Inset-New-Single-Port-Robotic-Technology_jpg)
Cleveland Clinic illustration showing single-port robotic instruments at work in a patient. The camera instrument works alongside the assistant instruments during surgery.
Advertisement
Advertisement

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Fixed-dose single-pill combinations and future therapies

Reproductive urologists publish a contemporary review to guide practice

Two recent cases show favorable pain and cosmesis outcomes

Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood

Proteinuria reduction remains the most important treatment target.

IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy

Oncologic and functional outcomes are promising, but selection is key