Novel drugs included in therapy
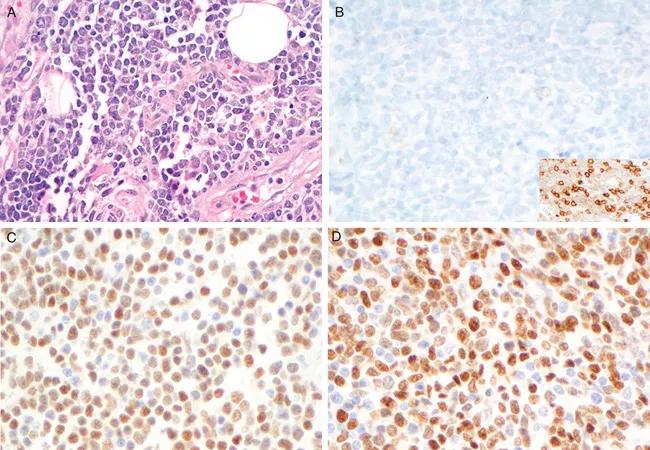
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4b9d41f8-92d9-4b57-92d2-29e6fad4975a/mantle_650x450_jpg)
mantle_650x450
Because there is no uniformly accepted frontline treatment regimen for mantle cell lymphoma (MCL), oncologists have long extrapolated treatment for the disease from treatment used for other aggressive B-cell lymphomas.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Until recently they used the chemotherapy drug combination of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). But MCL patients did not fare as well on this regimen as patients with other aggressive B-cell lymphomas, so oncologists added autologous stem cell transplantation (ASCT) to the treatment process.
The addition of consolidation ASCT after induction therapy with R-CHOP helped prolong survival in MCL patients, but there was still no cure. Patients in some high-risk subsets of MCL still weren’t surviving beyond a couple of years after diagnosis. Then the protease inhibitor bortezomib was approved for treating MCL patients, and in 2017, head-to-head clinical trials showed a regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin and prednisone (VR-CAP) was superior to R-CHOP for treating MCL patients.
With that in mind, investigators at two research centers — Cleveland Clinic and University of Washington/Seattle Cancer Care Alliance (SCCA) — began independently using VR-CAP, each developing the same modification by alternating it with rituximab + cytarabine during induction therapy for MCL patients. They hoped this would offer a superior pretransplant therapy than either previous treatment.
“There is a need for development of first-line regimens for MCL that incorporate novel therapies to improve outcomes, while not impairing stem cell collection,” says Brian T. Hill, MD, PhD, Director of the lymphoid malignancies program at the Cleveland Clinic Cancer Center, senior author of a study of the regimen published in Clinical Lymphoma, Myeloma & Leukemia.
Advertisement
Investigators retrospectively reviewed patients with newly diagnosed MCL, requiring therapy and potentially eligible for ASCT, who received modified VR-CAP/R+cytarabine as induction therapy starting in April 2015.
A total of 37 patients were treated with the modified VR-CAP regimen, 18 at SCCA and 19 at Cleveland Clinic. Most patients were intermediate- or high-risk.
To decrease neuropathy, a side effect of bortezomib, the investigators used attenuated dosing schedules and subcutaneous administration. Among 16 patients with available detail, 15 required platelet transfusions during high-dose cytarabine cycles, with a median of four units given (range, 0-16 units). Six of 8 patients required dose reduction of cytarabine at clinician discretion, starting at 3 gm/m2 , primarily due to thrombocytopenia. Ten patients experienced mild sensory peripheral neuropathy that did not impact bortezomib dosing. Cardiac failure during ASCT caused one patient death.
The researchers found that complete response to induction was achieved in 32 (86 percent) of 37 evaluable patients; two patients achieved partial response, and three had primary refractory disease. They also found that stem cell collection was successful in one attempt in 30 of 32 patients. They report that the median follow-up of survivors measured from start of treatment is 17.4 months, that five patients have progressed and that four have died (two from lymphoma, two from toxicity).
“This is a preliminary outcomes analysis of this approach,” Dr. Hill says. “Our overall survival and progression and progression-free survival at three years appear favorable compared with historic controls, but a limitation of this approach is that it is not a head-to-head comparison with our prior approach that did not incorporate bortezomib.”
Advertisement
Feature image: Cyclin D1-negative blastoid mantle cell lymphoma (all ×400). A, H&E; B, cyclin D1, inset CD5; C, CLO142; D, MRQ-58. Republished with permission from: Nakashima, MO et al. Utility and Diagnostic Pitfalls of SOX11 Monoclonal Antibodies in Mantle Cell Lymphoma and Other Lymphoproliferative Disorders. Applied Immunohistochemistry & Molecular Morphology. 22(10):720-727.
Advertisement
Advertisement
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches