Newly discovered signaling loop is shown to be druggable
An international research team has identified a novel signaling loop that shows potential as a therapeutic target in patients with glioblastoma. The previously undefined cellular pathway has been found to contribute to the spread and proliferation of glioma stem cells.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
While previous research has shown that activation of a protein called FGF2 (fibroblast growth factor-2) contributes to glioma stem cell self-renewal and tumor growth, it was not understood how. A new study co-led by Justin Lathia, PhD, of the Department of Cardiovascular and Metabolic Sciences, Cleveland Clinic Lerner Research Institute, identifies FGF2 as an important intermediary in a multistep, pro-cancer signaling loop and suggests that deactivating FGF2 may halt the growth and spread of glioblastoma.
Published in Cancer Discovery (2019 Aug 21 [Epub ahead of print]), the study is the first to identify FGF2 as a druggable target for glioblastoma, the most common primary malignant brain tumor. With standard treatment, the median survival for adults with glioblastoma is only between 11 and 15 months, and recurrence is very common. “New therapies are desperately needed,” Dr. Lathia says.
The research team found that a protein called ADAMDEC1 (a disintegrin and metalloproteinase domain-like protein decysin 1), which is secreted by glioma stem cells, breaks down the extracellular matrix, thereby enabling cancer cells to access key nutrients for their growth that otherwise would not be available.
One of those nutrients is FGF2. The researchers showed that ADAMDEC1 activates FGF2, which is found within the tumor microenvironment. Once activated, FGF2 selectively binds to and activates FGFR1 (fibroblast growth factor receptor 1), a receptor found on the surface of glioma stem cells.
Advertisement
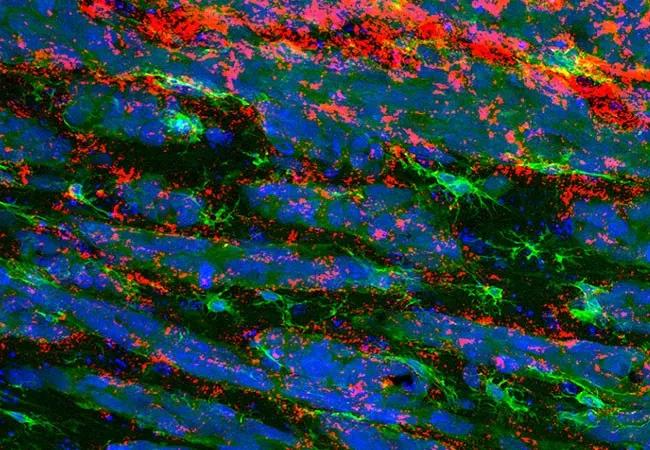
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1a7bc45d-941d-4fb4-8dc8-3648c258e8e4/19-NEU-3934-glioblastoma-ADAMDEC1-450x650_jpg)
Immunofluorescence staining showing the presence of ADAMDEC1 (red) in the tumor microenvironment. Immune cells are shown in green and nuclei in blue.
FGFR1, mediated through a few additional signaling cascades, plays two important roles in driving glioblastoma:
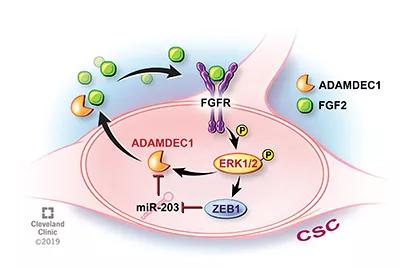
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/439c3457-171a-428f-8fcf-5cb8929c04aa/news_1566394881_jpg)
Illustration depicting the newly discovered cellular feedback loop. CSC = cancer stem cell. See text for expansion of other key abbreviations.
“These findings are exciting because they put forth a new paradigm for glioma stem cell regulation,” explains Dr. Lathia. “This pathway shows that the ability of glioma stem cells to access key nutrients in their surrounding microenvironment, by way of ADAMDEC1, is integral for their maintenance and spread. Finding a way to interrupt this feedback loop will be important for treating glioblastoma.”
While the investigators note that additional research is necessary, this study suggests that therapeutically targeting FGF2 may be the key to interrupting this cancer-driving loop.
Dr. Lathia is co-director of the Cleveland Clinic Center of Excellence in Brain Tumor Research and Therapeutic Development, which brings together traditional lab scientists with frontline physicians to advance care for patients with glioblastoma. The center is helping maintain Cleveland Clinic’s leadership in glioblastoma research, as Cleveland Clinic has one of the largest programs of active glioblastoma clinical trials in the country.
Advertisement
Dr. Lathia’s collaborators on this study include co-leader Dr. Florian Siebzehnrubl from the European Cancer Stem Cell Research Institute in Cardiff, Wales; Drs. Karl Holmberg and Karin Forsberg-Nilsson from Uppsala University, Sweden; Dr. Giorgio Colombo from the University of Pavia, Italy; Dr. Giulia Taraboletti from Istituto di Ricerche Farmacologiche, Italy; and Thomas McIntyre, PhD, from Cleveland Clinic Lerner Research Institute. Dr. McIntyre previously discovered, in the context of head and neck tumors, the role that ADAMDEC1 plays in driving cancer.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology