Low-dose, monitored prescription therapy demonstrates success

In the 34 years since the FDA approved topical minoxidil solution for hair loss, the brand Rogaine© has gained household-name status and established itself as a popular treatment for some forms of alopecia. Less well known but now growing in reputation is off-label, low-dose oral minoxidil, which is inexpensive and proving to be safe and quite effective on its own or in combination with topical treatments, says Cleveland Clinic dermatologist Wilma Bergfeld, MD.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Clinicians in Cleveland Clinic’s Department of Dermatology have been prescribing oral minoxidil for about three years with good results. The department currently is analyzing case data and photographs, but Dr. Bergfeld says, “Our general feeling is that we’ve been very successful.”
In the 1980s, Colorado dermatologist Guinter Kahn, MD, noticed that patients taking minoxidil for hypertension were experiencing hair growth as a side effect. His work on developing a minoxidil formula to be applied to the scalp led to the development of Rogaine, which was first approved for men, then later for women.
Early experiments in using minoxidil orally for hair loss resulted in unacceptable side effects, Dr. Bergfeld notes; the 10 mg dose was too high. Standard dosage for patients taking the drug for hypertension is 10 to 40 mg daily.
About six years ago, Australian dermatologist Rod Sinclair began investigating oral dosages as low as 0.625 and 1.25 mg for hair loss. The regimens were found to stop hair shedding and regrow hair abundantly in some patients while triggering few side effects. That body of evidence has continued to grow, says Dr. Bergfeld.
“The majority of our patients are on it for telogen effluvium, or shedding, and female or male pattern alopecia,” she says. “We start with 2.5 mg pills and have our patients split them to 0.625 or, more commonly, 1.25 milligrams a day. It has really been the best drug we ever had for hair growth.”
Dr. Bergfeld and her colleagues began using the regimen with patients who had hereditary and patterned hair loss, but have since started it as treatment for patients who have inflammatory alopecia as well. “The fact that the stem cells are there means the hair may be stimulated to grow, probably through the prostaglandin system,” she says.
Advertisement
Results may be visible within four to six months, but Dr. Bergfeld recommends waiting six to eight months to assess efficacy. Results for a given dose tend to be stable at one year. Increasing dosage or adding an additional treatment may be necessary.
In many cases, patients are adding low-dose oral minoxidil to existing topical regimens. “One of our patients had quite diffuse thinning. You could see her scalp all around the top of her head,” says Dr. Bergfeld. “She was on 5% topical minoxidil twice a day and we were using a little bit of Retin-A to increase the penetration. I put her on the oral and she did reasonably well – I could no longer see her scalp. But when I took her off the topical, we had a setback. So I tell patients that we’ll continue these therapies together for several months and then try to wean them off the topical treatment if we can.”
In a review of 1,404 Cleveland Clinic patients on the regimen, nearly 80% had experienced no adverse effects. Hypertrichosis, the most common side effect, appeared for 15.1%. Systemic adverse effects affected a total of 5.5%, and included light-headedness, fluid retention, tachycardia, headache, periorbital edema and insomnia. In most cases, patients experiencing side effects were able to continue treatment with an adjustment to dosage.
While results have been positive, Dr. Bergfeld stresses the importance of medical oversight.
“Minoxidil is not a drug that a patient can just go buy and take. It needs monitoring,” she says. “We have to ask the appropriate questions, and we have to be selective of the patient population for safety reasons.
Advertisement
“In our practice here at Cleveland Clinic, when a patient comes in with shedding, we must determine why, because shedding is a sign of internal disease or an event in life or a change in the chemistry of the body,” she adds. “We aren’t just giving it to everybody. We’re studying them first.”
Clinical follow-up includes a visual examination and photographs and routine blood work. Patients being treated for other conditions are likely able to take low-dose oral minoxidil for hair loss, but clinicians treating for those conditions must be made aware and approve of the regimen.
“Occasionally we have patients who have atrial fib or atrial flutter, and of course the cardiac specialists have to sign off on this drug in that case,” says Dr. Bergfeld. “We are walking hand in hand with our medical colleagues in our treatment of the patient.”
As the use of oral minoxidil for hair loss increase, so do the number of articles in popular press extolling its benefits. Still, without broader research, it is unlikely to earn official approval from the U.S. Food and Drug Administration.
“Is there a future for an investigative study sponsored by a pharmaceutical company?” says Dr. Bergfeld. “Probably not. The drug is already out there and it’s inexpensive.”
Still, the clinical results so far gave been “very good news,” she adds. “The growing literature continues to support the efficacy and safety of low dose oral minoxidil.”
Advertisement
Advertisement
Family history may eclipse sun exposure in some cases
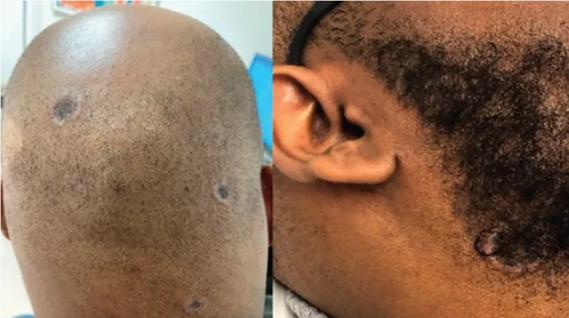
Consider secondary syphilis in the differential of annular lesions
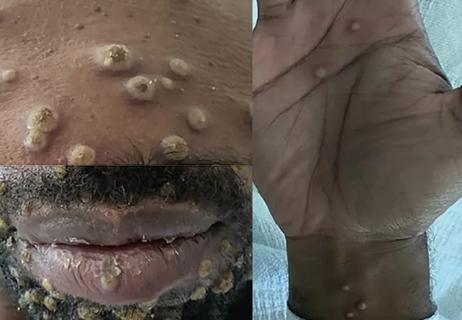
Persistent rectal pain leads to diffuse pustules

Two cases — both tremendously different in their level of complexity — illustrate the core principles of nasal reconstruction
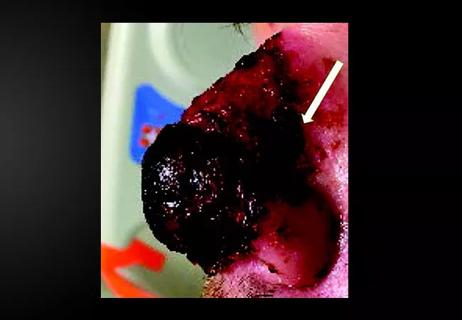
Stress and immunosuppression can trigger reactivation of latent virus

Antioxidants, barrier-enhancing agents can improve thinning hair
A case report of a thumbnail deformity