A hybrid approach
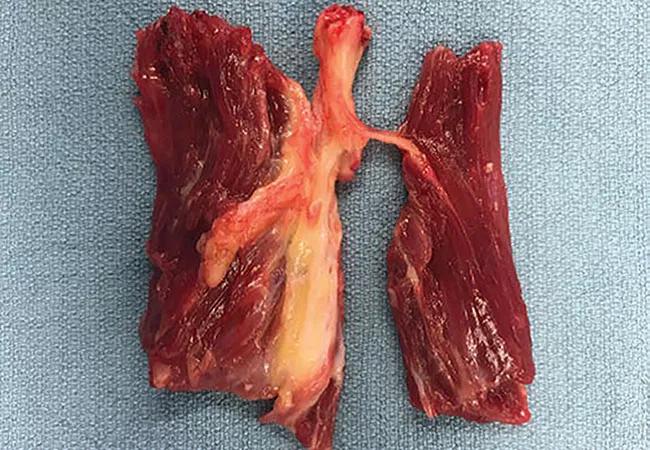
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3049834a-d3b5-4f62-8bbb-8c2e6f5591e6/21-PSX-2341408-CQD-Hero-1-650x450-1_jpg)
21-PSX-2341408-CQD-Hero-1-650×450
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Regenerative peripheral nerve interface (RPNI) is a novel approach to minimize the development of painful neuromas after limb amputations, such as below knee amputation (BKA) or above knee amputation (AKA). It is based on the premise that the proximal cut end of a nerve will attempt to grow and reinnervate a target; if not provided with an appropriate target for reinnervation, the growing nerve ending turns into a painful neuroma that can be severe and prevent prosthetic wearing.
RPNI involves harvesting a thin piece of muscle tissue (muscle graft) and wrapping it around the proximal cut end of the nerve in order to provide a target (denervated muscle) into which the nerve can grow.
A second technique for surgical management of neuromas is targeted muscle reinnervation (TMR), in which the surgeon coapts the transected nerve stump to an adjacent recipient motor nerve branch. Donor-recipient nerve size mismatch is an ongoing challenge with this technique. Both techniques have been successfully used separately and in a hybrid approach.
One hybrid approach that we have used successfully in multiple patients is targeted peripheral nerve interface (TPNI). TPNI involves harvesting neuromuscular units (a muscle graft innervated by a nerve branch) from the amputated limb and coapting these units to the proximal cut ends of nerves.
These neuromuscular units will guide axonal regeneration from the transected stump into target muscle grafts. TPNI avoids the mismatch issues of TMR and provides a more physiological interface (nerve-to-nerve coaptation) when compared with RPNI (nerve-to muscle-coaptation).
Advertisement
Two patients we have treated with this approach exemplify the efficacy of TPNI. The first was a 65-year-old woman with a 25-year history of refractory right ankle pain. The second patient was a 42-year-old man with recurrent osteomyelitis in his left ankle. Both patients had elected to undergo BKA.
In each patient, the first step was to identify the tibial nerve in the amputated leg and trace it distally as it branches into the muscles of the deep posterior compartment.
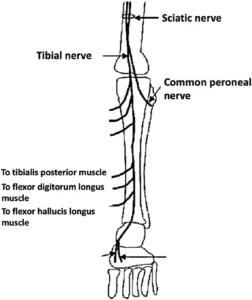
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/88f855bc-9618-42ff-85c0-a87a91bb1e5c/kao-1-252x300_png)
Anatomy of tibial nerve branches entering the muscles of the deep posterior compartment.
Then, the surgical team excised a 3 cm x 1 cm x 0.5 cm muscle graft around where the nerve branch penetrates the muscle, keeping the long axis of the muscle graft in parallel with the direction of the muscle fibers.
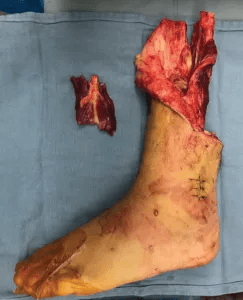
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6d974922-64e3-4152-8ac7-b337d2279095/kao-2-243x300_png)
TPNI harvested from the amputated portion of the leg.
The resulting TPNI resembles a standard RPNI but with a nerve branch penetrating the muscle graft centrally.
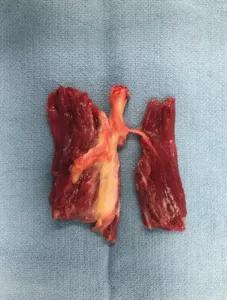
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4b0a7fee-e33a-4af6-b26b-8c25c4f57fa8/kao-3-227x300_png)
Distal nerve branch as it arborizes into the muscle. Two separate TPNI units can be harvested from this segment.
Next, the surgical team identified the proximal transected ends of the deep peroneal, sural, tibial, superficial peroneal and saphenous nerves in the BKA stump wound. Due to its larger caliber, the tibial nerve is usually split into three bundles, with each bundle individually coapted to a neuromuscular unit, thus forming three TPNI units. Similarly, the peroneal nerve is usually split into two bundles and individually coapted, forming two TPNI units.
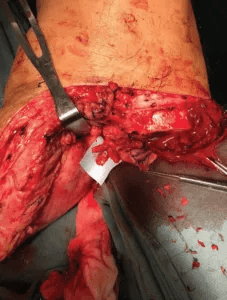
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6206cf78-2661-43d0-99f6-59ea273ffc6c/kao-4-227x300_png)
Splitting the proximal tibial nerve stump into multiple fascicles.
We used two 8-0 nylon sutures to coapt the nerves and applied fibrin glue to strengthen the nerve coaptation. Muscle graft suturing procedure closely follows the standard RPNI technique, which sutures the lateral edges using 5-0 Vicryl. Usually, we are able to obtain three to six TPNI from the amputated leg and can manage any remaining nerve stumps with standard RPNI.
Advertisement
At the three-month post-operative follow-up, both of these patients were ambulating independently with prosthetic limbs and had discontinued all narcotic pain medication that they had been using prior to surgery. They reported minimal phantom pain that did not interrupt their daily lives.
This hybrid approach has many benefits, the foremost of which is the ability of the nerve to regenerate along an intact nerve branch associated with the neuromuscular unit. This has the potential to better distribute the reinnervation pattern and thus produce less neuroma-related pain.
Our peripheral nerve surgery team, which includes myself, Megan Jack, MD, PhD, staff in the Neuromuscular Center, Joseph Styron, MD, PhD, staff in the Department of Orthopaedic Surgery, and Bryan Michelow, MD, staff in the Department of Plastic Surgery, is dedicated to the prevention and treatment of neuroma pain.
Dr. Kao is a hand and peripheral nerve surgeon at Cleveland Clinic.
Figures were originally published in PRS Global Open by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons and are republished without derivation under the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND).
Advertisement
Advertisement
Family history may eclipse sun exposure in some cases
Consider secondary syphilis in the differential of annular lesions
Persistent rectal pain leads to diffuse pustules
Two cases — both tremendously different in their level of complexity — illustrate the core principles of nasal reconstruction
Stress and immunosuppression can trigger reactivation of latent virus
Low-dose, monitored prescription therapy demonstrates success
Antioxidants, barrier-enhancing agents can improve thinning hair
A case report of a thumbnail deformity