Targeting DNMT1 shows promise in chemotherapy- and immunotherapy-resistant SCLC
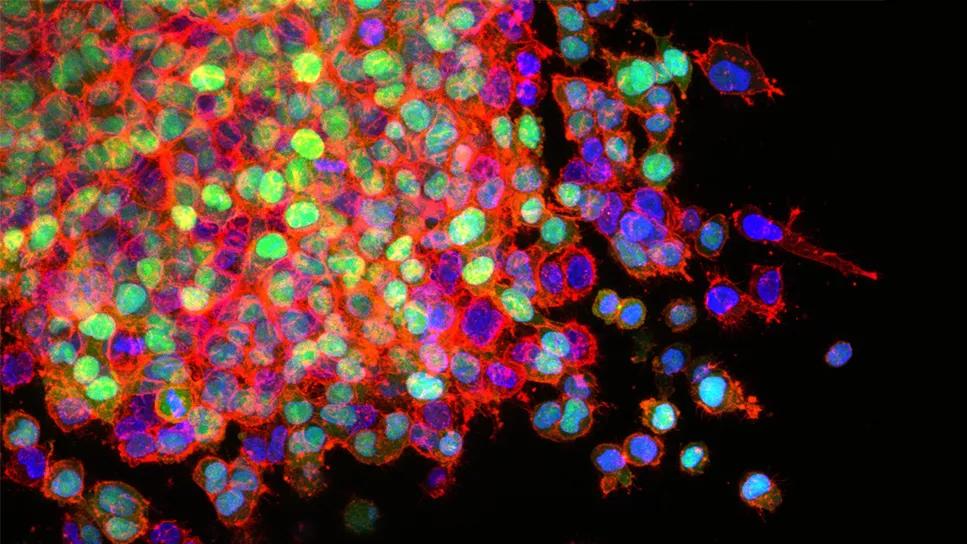
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer cases, and the number of cases are on the rise. Patients with SCLC typically present with advanced disease that rapidly resists cytotoxic chemotherapy, radiation therapy and immunotherapy. Most cases therefore have fatal outcomes. The current five-year survival for SCLC lingers around 5%, even with the addition of immune checkpoint therapy to standard platinum-based chemotherapy.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The key to treatment resistance is that SCLC cells delete the master switch ― the p53 gene ― that designates cells for cell suicide (apoptosis). Therefore, instead of dying after undergoing chemotherapy and radiation therapy-induced DNA damage, SCLC cells continue to divide and multiply.
SCLC cells are committed to a journey to become specialized neuroendocrine cells in the lungs, but then experience maturation arrest on this journey. That is, they fail to differentiate fully into normal neuroendocrine cells that focus on specialized neuroendocrine functions, and which no longer replicate.
In their latest study published in the August 2023 issue of Cell Reports, translational oncology researcher Yogen Saunthararajah, MD, and his team of collaborators investigated why SCLC cells get “lost” on their developmental journey and how this knowledge can be leveraged to develop new therapy for SCLC that is non-toxic and can work even when chemotherapy fails.
They found that the genes necessary for the final cell designation to specialized neuroendocrine functions cannot be “turned on” because they remain packaged away in the nucleus. They identified several enzymes mediating this packaging, one of which is DNA methyltransferase 1 (DNMT1), which is responsible for DNA methylation and is recurrently amplified in SCLC cells.
“DNMT1 keeps the genes for the end of the journey closed down in SCLC cells,” Dr. Saunthararajah explains.
In addition, investigators found that SCLC cells remove the Ten-Eleven-Translocation (TET2) gene, which functionally opposes DNMT1. Importantly, knocking down DMNT1 using siRNA or small molecular drugs led SLCL cells to resume their journeys to specialized pulmonary neuroendocrine fates, hence terminating replication naturally. This was demonstrated by unbiased comprehensive gene expression analyses as well as by separate analyses for signature genes, including GRP, NNAT, CHGB and SCG2.
Advertisement
Inhibition of DNMT1 and other packaging enzymes allowed “SCLC cells to complete journeys to become non-dividing specialized cells…we are fixing or healing, instead of trying, again and again, to force suicide in cells that have removed the suicide switch,” Dr. Saunthararajah says.
Experiments in murine models revealed that inhibiting DNMT1 with a combination of decitabine and 5-azacytidine extends survival with chemo-refractory and immune checkpoint inhibitor-refractory, p53-null, disseminated SCLC.
“The fact that SCLC cells are already committed to the journey to be pulmonary neuroendocrine cells can be leveraged into a new modality of non-cytotoxic therapy, by inhibiting DNMT1 and other packaging enzymes,” Dr. Saunthararajah notes. “Since the resulting cancer cell cycling exits do not require an intact suicide switch, and also spare normal cell divisions needed for health and lifespan, the clinical implications of these discoveries are significant.”
Historically, disseminated cancers have been treated by damaging DNA or other important life molecules using anti-metabolite chemotherapy and radiation, with the goal to try and activate the “guardian of the genome” p53 that designates cells for suicide. Although such an approach can cure cancers that have an intact p53-switch, this approach is inherently limited in cancer cells with a disabled p53 gene. These findings show that there is another way to terminate cancer growth, by allowing the cancer cells to complete the journeys to cell specialization they have already embarked on. “It is healing or fixing rather than destruction,” Dr. Saunthararajah says.
Advertisement
Targeting DNMT1 without causing cytotoxicity has already proven effective for treating the blood cancers myelodysplastic syndrome and acute myeloid leukemia. The drugs that work in blood cancers, unfortunately, do not distribute well into SCLC and other cancers of solid tissues. The investigators are therefore working on improving the available drugs. In parallel, they are also developing new agents, described in the publication.
“We are extending principles proven in blood cancers to other cancers like SCLCs, ovarian cancer, pancreatic cancer and gliomas, by making necessary improvements to existing drugs that engage ‘packaging enzymes’ as well as developing needed new drugs for this ‘fixing’ treatment paradigm,” says Dr. Saunthararajah.
“The only ultimate proof is clinical trials that show benefit,” he adds. “This data represents an important first step, and we are not stopping until we do these trials.”
Advertisement
Advertisement

Treatment assigned FDA review date in June 2025

Cleveland Clinic, the University of Minnesota and University of Cambridge receive $1M grant to develop point-of-care biosensor for early detection and treatment personalization

Hybrid treatment model helps improve cancer care access
Resection, radiotherapy or ablation?

Extent of baseline burden impacts progression-free and overall survival

Quantum computing being studied as a means to help improve predictive performance, accuracy
Young age, solid tumor, high uptake on PET and KRAS mutation signal risk, suggest need for lobectomy
New review published in Cancers suggests a synergistic benefit