What we know so far about the OAS-RNase L innate immune pathway
A paper published in Proceedings of the National Academy of Sciences shows for the first time how 5-azacytidine (AZA), a drug commonly prescribed to treat myelodysplastic syndromes and acute myeloid leukemia, causes cancer cell death. The paper also identifies several therapeutic targets that may help to amplify the drug’s already powerful disease-fighting effects.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
AZA is a DNA methyltransferase inhibitor, which prevents changes to the chemical structure and function of genes by a methylation. Preventing methylation increases the expression of double-stranded RNA (dsRNA).
The researchers, led by Robert Silverman, PhD, Department of Cancer Biology, elucidate how AZA, dsRNA and apoptosis are connected and mediated by the interactions of the OAS (2′,5′-oligoadenylate synthetase) family of genes and the antiviral protein RNase L.
Viruses often use dsRNA to replicate within a host, so the body typically launches an immune response to dsRNA. This immune response involves expression of interferons, as well as many interferon-stimulated genes, including the genes OAS1 to 3. dsRNA not only increases expression of OASs, but also activates these proteins. When active, OAS1 to 3 synthesize the molecule needed to activate a critical protein called RNase L, which causes apoptosis.
Implicating the OAS-RNase L pathway in this process provides a more comprehensive understanding of how AZA and dsRNA prevent the proliferation of cancer cells, which may prove helpful in other types of cancers, too. It also offers important insights about how a host antiviral protein can, under some conditions, function against cancer cells. Engineering and training the body’s cells to to fight cancer is a growing area of interest among researchers.
Understanding this pathway has led to the identification of several actionable targets that can help increase AZA’s cytotoxic effects, like using ionizing radiation or silencing specific genes or their encoded proteins, such as ADAR1 (adenosine deaminase acting on dsRNA 1) or PDE12 (phosphodiesterase 12). Future studies will be important to test and validate these targets.
Advertisement
Shuvojit Banerjee, PhD, is first author on the study, which was supported in part by the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health.
A VeloSano award, Cleveland Clinic’s flagship philanthropic initiative to advance cancer research, also helped make this project possible, demonstrating the value of early philanthropic funding to generate the data needed to secure larger extramural funding.
TW/FB: We now know how 5-azacytidine (AZA) induces cancer cell death, thanks to a deepened understanding of the OAS-RNase L pathway.
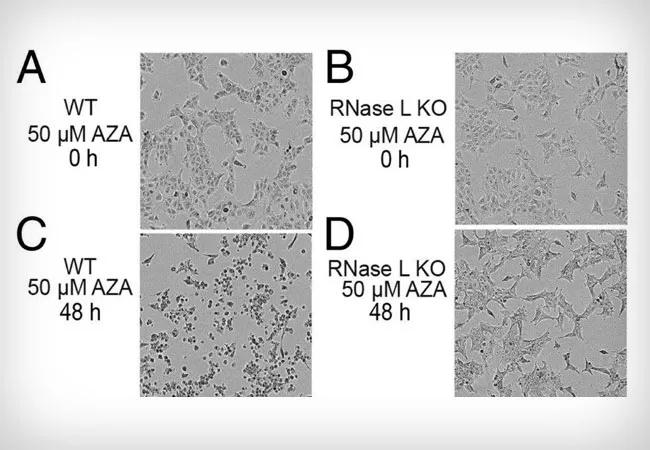
Feature image: RNase L mediates cell death in response to AZA treatment of A549 cells. (A–D) Phase-contrast images of (A and C) WT and (B and D) RNase L KO A549 cells at 0 and 48 h after adding AZA (50 μM) to media. Originally appeared here as part of Figure 1, copyright held by authors.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology