Combination of olaparib and carboplatin results in complete durable response for a patient with BRCA2 and “BRCAness” mutations
Originally diagnosed with stage 4 triple-negative invasive ductal carcinoma in July 2013, a woman in her early 60s sought a second opinion from Cleveland Clinic Cancer Center after progressing subsequent to third-line chemotherapy. After initiating a combination therapy of the poly-ADP ribose polymerase inhibitor olaparib along with carboplatin, she had a complete and durable response. This is an unusual occurrence, as it is rare for a patient with advanced triple-negative breast cancer that has metastasized to the brain to achieve a complete response in a fourth line of treatment and stay off chemotherapy for more than seven years.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The patient had an initial diagnosis of stage 4 triple-negative invasive ductal carcinoma of the left breast, with multiple lesions in the lung in July 2013. She was treated with dose-dense doxorubicin and cyclophosphamide for four cycles, followed by weekly paclitaxel. Her surgeon performed a simple mastectomy in January of the following year. In July 2014, progression occurred in the lung. MRI imaging of her brain also revealed a 3-cm mass in the left frontal lobe.
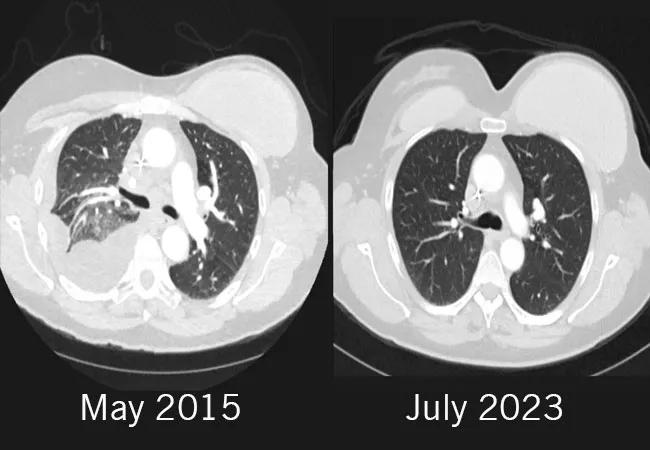
The left frontal area was treated with surgical resection and Gamma Knife surgery in September 2014. Her medical team started her on Gemcitabine, and later transitioned her to capecitabine as part of a clinical trial. By May of 2015, the treatment was halted due to disease progression. She was considered for a clinical trial of olaparib monotherapy, but was ineligible. Due to the lesions on her lung, she was experiencing profound shortness of breath and chest pain. By that time, her tumor progressed on three lines of therapy in the metastatic setting, and her performance status was declining due to shortness of breath and intense chest pain due to large lesions in the lung.
The patient sought care with Chair of the Cleveland Clinic Cancer Center’s Department of Hematology and Medical Oncology Jame Abraham, MD, FACP. Due to the patient’s family history of breast cancer and her diagnosis with triple-negative breast cancer, Dr. Abraham ordered next-generation sequencing of the tumor and germline testing for the BRCA mutation. When her germline testing resulted in BRCA 2 mutation, Dr. Abraham knew he had the option to treat the patient with a PARP inhibitor. Interestingly, her tumor germline testing was positive for PTEN, BRCA2, MYC and TP53. However, PARP inhibitors such as olaparib was not approved for breast cancer in 2015. .
Advertisement
Based upon promising phase 2 data that supported the use of the combination of the PARP inhibitor olaparib and the chemotherapy carboplatin, Dr. Abraham chose this course of treatment. He initiated off-label use of olaparib 300 mg twice a day, with carboplatin added on days 1 and 7. “The combination of olaparib and carboplatin demonstrated remarkable effectiveness in this patient with germline mutation”,” says Dr. Abraham. “By the third cycle of treatment, her scans showed an excellent response and the patient had remarkable improvement in her symptoms.”
Side effects included grade 3 thrombocytopenia with a platelet count of 37,000/uL. After holding the therapy for two weeks, the patient’s side effects improved. After six cycles of carboplatin, she was able to stop chemotherapy and continue solely on olaparib 300 mg twice a day. She tolerated the treatment well, with minor gastrointestinal symptoms. The lung lesions completely disappeared. She soon became asymptomatic and resumed working full-time and started playing tennis. Her symptoms completely resolved.
A small lesion appeared on the patient’s brain in 2020, resulting in a subsequent Gamma Knife surgery, and a switch of olaparib to the PARP inhibitor talazoparib. CT scans as well as brain scans are performed every six months.
Nearly 10 years after initial diagnosis, the patient has no evidence of active disease. “This case illustrates the importance of germline testing as well as next-generation sequencing in metastatic breast cancer,” says Dr. Abraham.
Advertisement
Based upon randomized trials where the PARP inhibitors olaparib and talazoparib were found to be superior than treatment of physicians choice, the FDA has approved the use of these agents in metastatic breast cancer with germline mutations in 2018.
Advertisement
Advertisement

Real-world results reporting aims to make treatments safer and more effective

Obstructing key protein allows for increased treatment uptake for taxane chemotherapy

Polygenic risk score could help predict who will develop this aggressive breast cancer

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Reconsidering axillary lymph node dissection as well as depth of surgical margins

Ultra-Hypofractionated Whole Breast Irradiation and Partial Breast Irradiation Reduce Many Toxicities

Robotic-Assisted Procedures Offer Breakthroughs in Lymphedema Treatment After Breast Cancer Surgery

Best practices for reducing toxicities