By Mistyann-Blue Miller, MD, Interventional Cardiology, Cleveland Clinic Indian River Hospital
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Note: This article is reprinted from Cardiac Care Publication News From Cleveland Clinic in Florida.
Between 2.5 and 6 million people in the United States live with atrial fibrillation (AF). Patients diagnosed with AF are at five-fold increased risk of ischemic stroke compared to those without AF. Treatment with oral anticoagulants including direct oral anticoagulants and the vitamin K antagonist, warfarin, is the standard of care for these patients to prevent thrombus formation in the left atrial appendage (LAA), where more than 90% of blood clots form due to stagnant blood flow.
Unfortunately, these agents confer a significantly increased risk of both major and minor bleeding events, which range from bruising or bleeding from everyday abrasions to catastrophic gastrointestinal bleed or intracranial hemorrhage. Moreover, labile international normalized ratio (INR), medication noncompliance, affordability of medications, and lifestyle and occupational habits have made it increasingly difficult for patients to adhere to their treatment regimen.
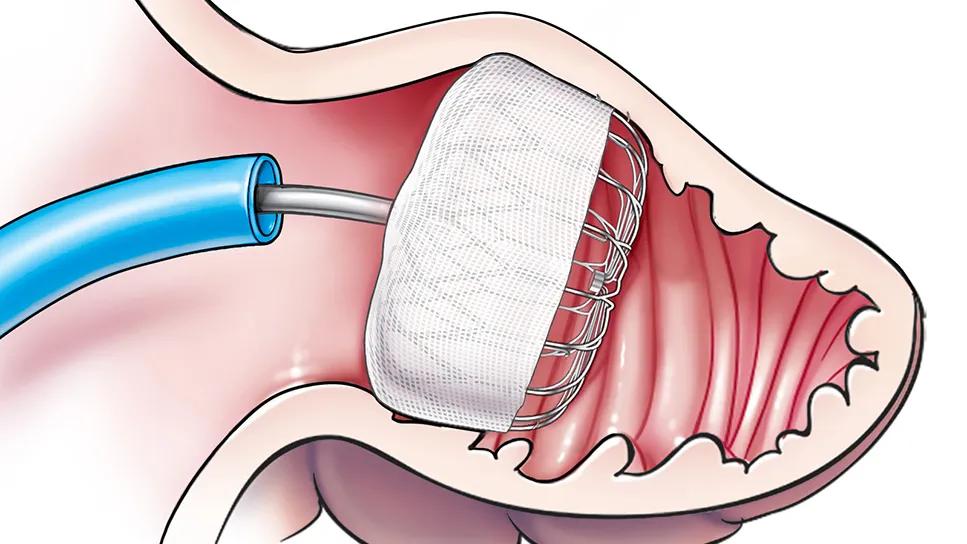
Left atrial appendage occlusion (LAAO) is a procedure developed to address the needs of many patients with nonvalvular AF at high risk for ischemic events, who encounter obstacles to taking oral anticoagulants (OAC). The most widely implanted LAAO devices include the WATCHMAN FLX™ and Amplatzer™ Amulet™ devices. Both occluders are designed to fit into the LAA, thus excluding it from systemic circulation and preventing the formation of thrombus and potential embolization.
Advertisement
Under transesophageal echocardiography (TEE) and fluoroscopic guidance, the device is delivered via catheter and sheath through a small transseptal puncture that is made from the right atrium into the left atrium by the implanting physician. Once the appropriate-sized device is selected and several intraprocedural safety parameters are met, the occluder is released from the delivery catheter and deployed securely in the LAA.
At Cleveland Clinic Indian River Hospital, patients stay one night or less in the hospital post-implant.
If the WATCHMAN device is used, patients remain on their OAC in addition to 81 to 100mg aspirin for at least 45-days post-procedure, at which time a follow-up TEE is performed to confirm device stability, adequate endothelization, and to ensure no peri-device leak.
If these criteria are met, OAC is discontinued, and the patient is treated with dual antiplatelet therapy with baby aspirin and P2Y12 inhibitor (e.g., clopidogrel) until six months post-implantation. At six months the P2Y12 inhibitor is discontinued and aspirin monotherapy is continued indefinitely. In the Amulet studies, patients were treated only with dual antiplatelet therapy post-device implant, eliminating the need for anticoagulants.
Potential complications from the LAAO implant include device embolization, pericardial effusion and ischemic stroke. However, implantation of the WATCHMAN FLX device is proven safe and effective with high procedural success and very low complication rates across several trials. Clinical studies from the American College of Cardiology’s LAAO Registry™ also have demonstrated overwhelming success of this procedure in real-world clinical practice across the United States, with device success rate of 98% and complication rate of 0.37%, and randomized clinical trials comparing both devices have reported similar safety and effectiveness at preventing ischemic strokes.
Advertisement
Implantation of the LAAO device is clinically safe and effective in preventing ischemic stroke in patients with nonvalvular AF and is a viable alternative for those who are at high bleeding risk or who have difficulty adhering to OAC treatment regimens.
Advertisement
Advertisement

Nonthermal technique reduces bleeding and perforation risk

Standardizing a minimally invasive approach for Barrett’s Esophagus and Esophageal Cancer

PSMA-targeted therapy for metastatic prostate cancer now offered at Cleveland Clinic Weston Hospital

Nationally recognized urologic oncologist offers vision for growth, innovation, and excellence

Noninvasive modality gains ground in United States for patients with early-to-moderate disease

Cleveland Clinic Weston Hospital’s collaborative model elevates care for complex lung diseases

Interventional pulmonologists at Cleveland Clinic Indian River Hospital use robotic technology to reach small peripheral lung nodules

Trained in the use of multiple focal therapies for prostate cancer, Dr. Jamil Syed recommends HIFU for certain patients with intermediate-risk prostate cancer, especially individuals with small, well-defined tumors localized to the lateral and posterior regions of the gland.