APE2 may hold the key to reducing cisplatin toxicity
Cisplatin chemotherapy is standard of care for many cancers. The drug is administered in a one-size-fits-all approach and can be toxic to the kidneys, leading to discontinuation of treatment or a reduction in dose.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Scientists in the Department of Cancer Biology in Cleveland Clinic’s Lerner Research Institute recently found that inhibiting a protein molecule known as apurinic/apyrimidinic endonuclease 2 (APE2) helped lessen renal damage from cisplatin chemotherapy in a preclinical model. Now they have received a five-year, nearly $2.6 million grant from the National Cancer Institute to further advance their research.
“We think APE2 is a novel target with potential utility for treatment or prevention of this unfortunate but common side effect,” says Jianjun Zhao, MD, PhD, co-senior author of the study, which was published in Cancer Research earlier this year, and lead researcher on the grant.
Cisplatin damages the genetic material in rapidly dividing tumor cells, effectively interrupting DNA replication and preventing the spread of cancer cells. Damage to non-cancer cells can occur while the body works to metabolize and excrete the drug.
“Although the body excretes 50% to 80% of the administered cisplatin within a day or so, some remains in the kidney, where it damages specific cells in the nephron, which removes waste from the blood before it is converted to urine,” Dr. Zhao explains.
In his team’s recent study, which involved C57B6J mice, Dr. Zhao and his team observed that APE2 levels in mitochondria within proximal tubule cells rose following administration of cisplatin. Further analysis revealed that this occurred because APE2 binds with myosin heavy-chain 9 (MYH9) protein in the mitochondria (Figure).
Advertisement
“We believe that this abnormal APE2-MYH9 binding compromises mitochondrial function and causes cells to die, resulting in the type of kidney damage commonly associated with cisplatin treatment,” says Dr. Zhao.
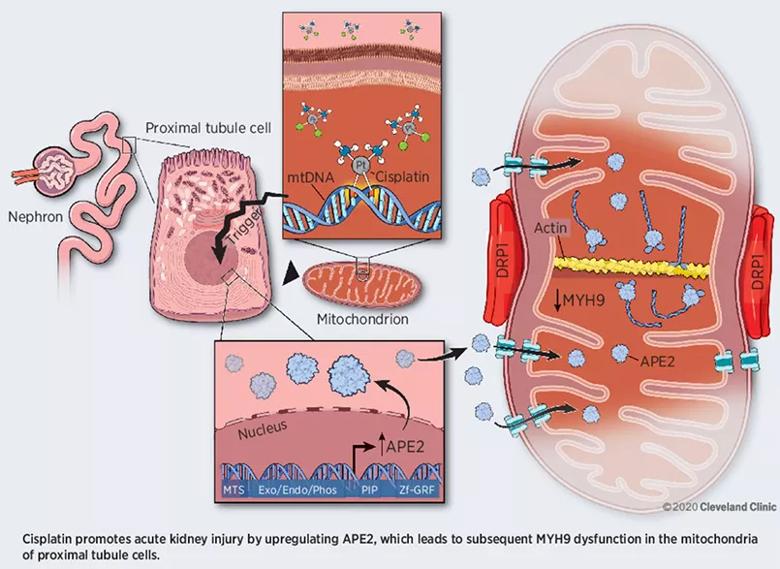
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ef25a866-0bc3-4756-9fd9-629dc4253c14/21-CCC-2330110-INSET-1_jpg)
Figure. Proposed model by which APE2 binding to MYH9 in mitochondria induces acute kidney injury. Reprinted from Hu et al., Cancer Res. 2021;81:713-723. ©2020 Cleveland Clinic.
Human MYH9-related disorder is caused by mutations in MYH9 that eventually lead to nephritis, macrothrombocytopenia and deafness — a collection of symptoms that mirrors the toxicity profile of cisplatin.
Dr. Zhao and colleagues found that genetically knocking out APE2 in the mice treated with cisplatin significantly reduced cisplatin-induced acute kidney injury, suggesting that pharmacologically targeting the protein may be a viable approach to treating the condition in humans.
Dr. Zhao looks forward to continuing to pursue this line of investigation with support from the new National Cancer Institute grant. In their next phase of study, his team will continue work to develop a reliable mouse model of chemotherapy-associated kidney damage to study disease processes and test potential therapeutics. They will now compare the molecular features of their model to those observed in patient samples and study how APE2 interacts with MYH9 and other proteins to promote acute kidney injury.
Using their model, the researchers will also test the ability of two compounds to suppress APE2 expression. “Based on our previous research, we know that using genetic engineering technology to completely prevent APE2 expression had positive effects,” says Dr. Zhao, “but now we need to find feasible ways to prevent the protein’s expression or activity in patients through drug development.”
Advertisement
The researchers will test two different approaches to inhibit APE2 activity in a specific type of kidney cells. They will test whether the drug candidates can overturn kidney damage in their preclinical model of cisplatin-induced acute kidney injury, as well as if they can prevent kidney injury from occurring in preclinical lung cancer models treated with cisplatin.
“There are two ways to improve outcomes for cancer patients: by developing new therapeutics or by finding ways to optimize the ones we have,” Dr. Zhao concludes. “We hope this line of research will lead to a method of treating or preventing this unfortunate side effect of a valuable chemotherapeutic agent. This would make it possible to continue administering cisplatin without making adjustments that may compromise the drug’s cancer-fighting potential.”
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology