Integrates genetic and clinical data to distinguish from GEFS+ and milder epilepsies
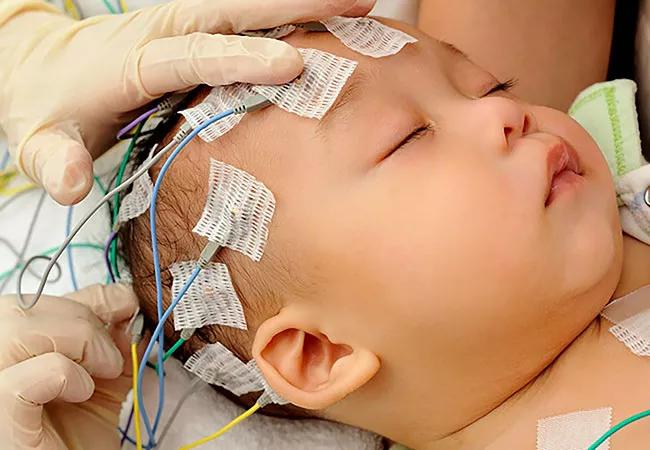
An international research team co-led by Cleveland Clinic’s Lerner Research Institute has developed and validated a prediction model to aid in the early diagnosis of Dravet syndrome, a severe epilepsy characterized by drug-resistant seizures, intellectual disability and high mortality that begins in infancy. The model and its validation were recently published in Neurology (2022;98[11]:e1163-e1174).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The majority of Dravet syndrome cases are caused by variants in the SCN1A gene, which codes for a sodium channel protein involved in the regulation of brain cell activity. However, SCN1A variants also have been linked to other epilepsies, such as genetic epilepsy with febrile seizures plus (GEFS+), a much milder syndrome that does not affect cognition.
“Dravet syndrome initially appears clinically similar to GEFS+, so clinicians must wait until distinguishing symptoms, such as developmental delay, emerge before making a definitive diagnosis,” says the study’s co-senior author, Dennis Lal, PhD, assistant staff in Cleveland Clinic’s Genomic Medicine Institute. “However, this reliance on clinical observations alone means they often miss the opportunity for early intervention, which is critical to achieving the best patient outcomes.”
To address this challenge, the researchers built a statistical model that calculates the probability of developing Dravet syndrome versus GEFS+ by integrating clinical and genetic data from an international cohort of 1,018 patients with SCN1A-related Dravet syndrome or GEFS+. They found that the model outperformed any previous or alternative models, indicating its utility as a clinical decision-support tool that can help clinicians differentiate between the disorders at an early stage.
Specifically, the model accounts for age at seizure onset, which is the earliest clinical symptom that can be reliably assessed in infancy, and the likelihood a given SCN1A variant confers risk for Dravet syndrome (i.e., genetic score). A higher genetic score denotes an increased risk of Dravet syndrome.
Advertisement
“Consider the example of a 9-month-old infant presenting with recurrent febrile seizures and a pathogenic SCN1A variant,” Dr. Lal explains. “Based on the age at onset alone, a clinician would likely estimate the risk of Dravet syndrome to be moderate, or around 50%. But with the additional information that the infant carries a variant with a high genetic score, the estimated risk could increase to more than 90%. Alternatively, a low genetic score might reduce that risk to less than 10%.”
The model, which uses data easily accessible to clinicians treating any young infant presenting with a pathogenic SCN1A variant, can be accessed for free online. The model is for education purposes only and should not substitute for medical or professional care.
“Our model can assist in the early and accurate prediction of whether an infant or young child with an SCN1A variant will develop Dravet syndrome or the milder GEFS+, which is vital information needed to make the best decisions regarding patient management and treatment planning,” Dr. Lal concludes. “In addition, our approach for developing a clinical decision-support algorithm is generalizable and can be applied to many other genetic disorders where genetic and clinical data are available.”
The study was supported by the Dravet Syndrome Foundation, the German Federal Ministry of Education and Research, the National Institute of Neurological Disorders and Stroke and Dravet Syndrome UK.
Advertisement
Advertisement

Study identifies Ketorolac as a potential repurposable drug

The relationship between MTHFR variants and thrombosis risk is a complex issue, but current evidence points to no association between the most common variants and an elevated risk

One-time infusion of adenovirus-based therapy is designed to restore heart muscle function
Cleveland Clinic researchers receive $2 million grant from the National Institutes of Health

New Cleveland Clinic fellowship fosters expertise in the genetics of epilepsy

First-of-kind study aims to enable prevention of brain disorders before symptoms appear

Advanced genomic research techniques show potential to treat a variety of conditions

TNFRSF1B gene variant associated with slower progression, offering potential drug target