Research project will leverage insights into neural circuits to advance DBS technology
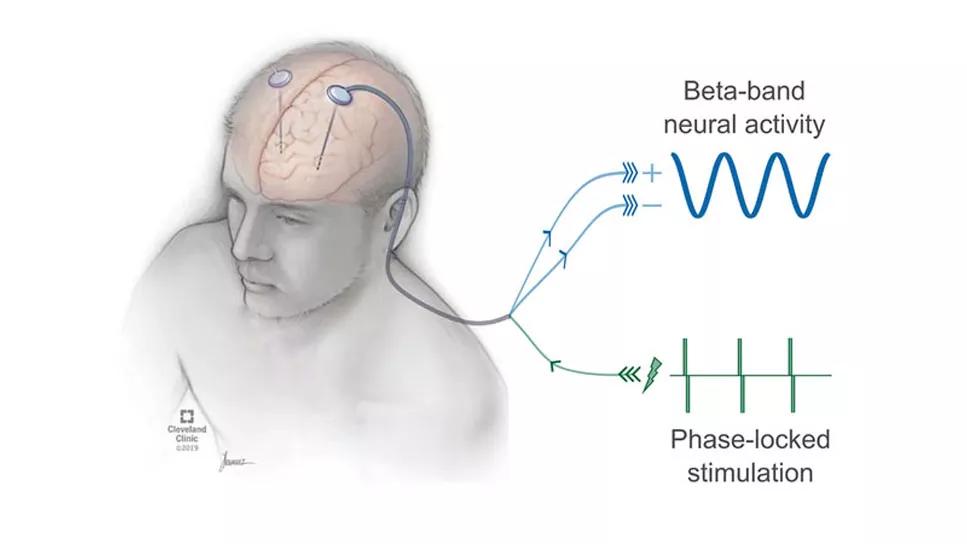
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8f53ef8f-eaa1-4b31-a99c-668578dd4fb6/23-NEU-40859124-CQD-Hero-967x544-1_jpg)
23-NEU-4085912-CQD-Hero1-Whole-650×450
Although deep brain stimulation (DBS) has been used for more than two decades to treat the motor symptoms of Parkinson’s disease (PD), the disease’s causal mechanisms and the precise way in which DBS affects the neural substrate to mitigate symptoms remain unknown.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Now, an enterprising Cleveland Clinic research project aims to identify the specific neural dynamics that underlie PD movement disruptions and use that knowledge to develop individualized, feedback-responsive “closed-loop” DBS technology to improve treatment.
The project is funded by a new five-year, $3 million grant from the National Institute of Neurological Disorders and Stroke.
“Our overall goal is to understand Parkinson’s disease better, efficiently program and adjust DBS systems based on each patient’s neurophysiological signals, and ultimately minimize the variability of outcomes and DBS costs, so this therapy is effective and accessible for more patients,” says the project’s principal investigator, David Escobar, PhD, Director of Cleveland Clinic’s Neural Dynamics and Modulation Laboratory.
“The long-term intent is a more intelligent, adaptive closed-loop DBS system that uses the patient’s own electrophysiology to make stimulation changes in real time,” adds co-investigator Kenneth Baker, PhD.
“Today, when we implant DBS, the pacemaker runs continuously,” says neurosurgeon Andre Machado, MD, PhD, Chair of Cleveland Clinic’s Neurological Institute and a co-investigator on the research project. “Regardless of what the patient is doing, it’s pushing the same amount of stimulation. The problem is that’s not how the brain functions. Our brain modulates in activity depending on what we are thinking, if we’re about to move and if we’re about to change what we’re doing.
“What Drs. Escobar and Baker are attempting is to learn the language of the brain so that DBS one day can be dependent on what the brain is trying to do,” Dr. Machado says. “They’re also trying to understand what happens in the brain in Parkinson’s disease, because that tells us what steps to take next.”
Advertisement
The new research project targets the electro- and pathophysiology of the basal ganglia in PD.
Although basal ganglia deterioration and dysfunction characterized by dopamine depletion are hallmarks of the disease, many specifics of this process and exactly how it contributes to patients’ movement problems remain unclear.
Within the past 20 years, an evolving conception of basal ganglia disruption in PD has emerged. It is based on electrophysiological studies of animal models of parkinsonism and patients with PD that show changes in the synchronization between and within neuronal populations.
Specifically, DBS intra- and postoperative electrode recordings of local field potentials (LFPs) indicate that clusters of basal ganglia neurons in the subthalamic nucleus (STN) and globus pallidus interna (GPi) exhibit elevated oscillatory activity with increased synchrony. Since synchronization boosts post-synaptic efficacy, these observed changes are likely to influence the basal ganglia’s projection targets such as the thalamus.
The abnormal oscillatory activity in patients with PD occurs in the beta frequency band (11-35 Hz) throughout the basal ganglia-thalamocortical circuit (BGTC) and is associated with bradykinesia and rigidity. Treatment with the dopamine precursor levodopa decreases patients’ beta band synchronization. This reduced oscillatory synchrony is correlated with improved motor symptoms.
Since the BGTC plays a major role in motor control, it is reasonable to infer that elevated, tightly coupled beta oscillations in this circuit hinder its information-processing capabilities, perhaps by degrading neurons’ information-carrying capacity. However, there is no definitive evidence to date of a causal relationship between the elevated, synchronous LFP oscillations and PD motor symptoms.
Advertisement
Current DBS systems deliver continuous high-frequency (~130 Hz) electrical pulses to the STN or GPi that are capable of suppressing beta-band oscillations in PD patients. Patients’ motor function improves with stimulation. But it is unknown whether a decrease in beta-band activity results in motor symptom reduction or is a related epiphenomenon.
Determining if a causal link exists between elevated, synchronized beta-band oscillations and PD motor symptoms, as well as probing how DBS relates to therapeutic outcomes, requires a new kind of neuromodulation tool that can detect and precisely control oscillatory activity in the brain in real time without using high-frequency stimulation.
Dr. Escobar led development of such a technology, called evoked-interference closed-loop DBS (eiDBS). It analyzes LFP recordings and delivers stimulation with the exact magnitude and timing (stimulation locked to the oscillations’ phase) to evoke a resonant response in a targeted neuronal population, predictably amplifying or suppressing spontaneous beta-band oscillations in the circuit via constructive or destructive interference. The technology has been used to successfully modulate beta oscillations in proof-of-concept experiments in nonhuman primates and several PD patients.
“Most physical systems have a natural resonance frequency,” Dr. Escobar explains. “The neural circuits in Parkinson’s, we believe, resonate in the beta frequency.”
The elevated, synchronous LFP oscillations characteristic of PD might be a byproduct of the BGTC’s resonance, which amplifies the oscillations and enables them to propagate. “We have preliminary data suggesting that the circuit’s resonance is decreased or its oscillatory damping is increased when we give medication to patients,” he says. With neural recordings, “we can see the timing and amplitude of those oscillations. When we stimulate with eiDBS in the same resonant frequency, we put the oscillations in phase and amplify them. When we stimulate out of phase, we suppress the oscillations.”
Advertisement
Using eiDBS, Drs. Escobar and Baker and their colleagues will investigate whether controlled amplification or suppression of beta oscillations in the STN of PD patients produces changes in bradykinesia and rigidity (Figure), and how the administration of levodopa affects these stimulation-evoked oscillations.
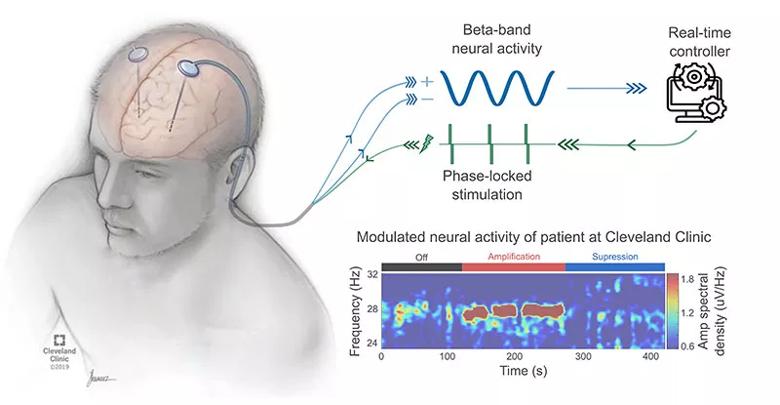
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4052ffc5-1ef2-4e5f-9fdc-3b06b5594980/23-NEU-4085912-CQD-Inset-800x415-1_jpg)
Figure. Schematic of closed-loop evoked interference deep brain stimulation (eiDBS). In real time, the eiDBS system delivers electrical pulses with precise amplitude and timing to evoke oscillatory responses that modulate spontaneous oscillations via constructive or destructive interference. The time-frequency color map represents the power of electric potentials measured with a DBS lead implanted in the subthalamic nucleus of a patient with PD. This color map shows how beta-band neural activity, thought to be linked to the manifestation of slowness of movement and rigidity in PD, is suppressed or amplified using eiDBS.
They also will aim to identify which neuronal pathways connected with the STN need to be stimulated to produce resonant responses in the BGTC, a key to understanding the spatiotemporal circuit dynamics underlying the generation of beta oscillations and the manifestation of PD motor dysfunction.
“We’re trying to determine if we can write information into one node of the brain and achieve a desired response,” Dr. Escobar explains. “Our rationale is that the STN is like a hub, and when we stimulate there, the circuit’s resonance will propagate the signal and help suppress spontaneous oscillations linked to Parkinson’s disease. Our aim is to gain a new understanding of circuit resonance as a phenomenon in the brain. The potential is to target that property of the circuit directly in the future.”
Advertisement
With improved knowledge of PD circuit dynamics, the researchers hope to optimize eiDBS for maximum modulatory effects — by delivering directional stimulation that activates relevant pathways to neutralize beta oscillations while minimizing activation of uninvolved pathways to reduce the potential for side effects.
To test the effects of eiDBS neuromodulation on PD motor symptoms, the researchers will conduct a five-year clinical trial involving 25 patients with PD who are scheduled to undergo DBS implantation surgery and whose bradykinesia or rigidity is mild to severe on the Unified Parkinson’s Disease Rating Scale.
Before surgery, the patients will undergo brain imaging studies using high-resolution MRI. That imaging will allow the researchers to build patient-specific 3D anatomical models of the STN and its interconnections, helping them determine which neuronal pathways need to be stimulated to evoke resonant responses in the STN and BGTC and bolstering their understanding of circuit dysfunction in PD.
During surgery, the DBS leads will be placed in the STN. Lead extensions will be temporarily externalized through patients’ contralateral scalp or shoulder and connected with the eiDBS system. Lead externalization is necessary because commercially available implantable DBS systems capable of recording LFP data cannot implement the eiDBS operating algorithms or deliver stimulation in the required beta-band frequencies during LFP recording.
Over several days, the researchers will conduct experiments to address the study’s key aims.
Initially, they will quantify patients’ baseline off-medication motor symptom severity, using electromyography and inertial sensors to evaluate bradykinesia during movement tasks, and a robotic system the investigators developed that captures rigidity measurements while manipulating patients’ elbows and wrists.
The researchers will use neural recordings of each patient to determine the eiDBS stimulation parameters that will maximize amplification or suppression of LFP beta oscillations. They will then test whether either type of stimulation affects patients’ motor symptoms.
The presumption is that amplification will increase bradykinesia and rigidity, while suppression will decrease them. The researchers also hypothesize that levodopa administration will decrease the amplitude of stimulation-evoked oscillations, correlating with reduced motor symptoms — an outcome that can be explained by a reduction in the BGTC’s resonance.
“If there is an effect, we can infer that there is a causal relationship” between STN beta oscillations and PD motor symptoms, Dr. Escobar says. Lack of an effect would imply that modulation of beta oscillations in the STN alone is not sufficient to mitigate bradykinesia and rigidity. However, a negative outcome would not disprove causality, he says, since unknown confounding factors could influence the result.
Either way, insights from the trial will help guide the team’s future research. The results will inform the researchers’ efforts to devise neurophysiology- and imaging-based programming strategies for DBS systems and to develop implantable devices that implement eiDBS.
“The goal of our research ultimately is to reduce the cost of DBS and narrow the variability of results across patients and medical centers,” Dr. Escobar says. “This project will be a step toward truly personalized eiDBS for Parkinson’s disease. We also hope to establish eiDBS as a research tool and potential therapy for other brain conditions believed to derive from pathological oscillatory activity, such as essential tremor.”
Dr. Escobar credits the progress in his DBS research to collaboration among Cleveland Clinic experts in a wide array disciplines, including neurosurgery (Andre Machado, MD, PhD; Sean Nagel, MD; and Richard Rammo, MD), neurology (James Liao, MD, PhD, and Benjamin Walter, MD), radiology (Stephen Jones, MD, PhD, and Mark Lowe, PhD), neuroscience (Kenneth Baker, PhD, and Brett Campbell, PhD), quantitative sciences (Xuefeng Liu, PhD) and biomedical engineering (Dr. Escobar’s department). He also believes the advanced technology at Cleveland Clinic — including 7-tesla MRI and magnetoencephalography — will help further advance research into PD and DBS. “We have all this infrastructure and a phenomenal team that can do really unique things to advance the understanding Parkinson’s disease,” he says. “Cleveland Clinic is a great place to do clinical research.”
The eiDBS project is part of a comprehensive effort by Cleveland Clinic to improve treatment for PD, from genetic studies to research involving gait problems, the benefits of exercise, the potential application of immunotherapies, predictive biomarkers for PD dementia, the neural basis for cognitive decline and numerous other areas.
Advertisement
Various AR approaches affect symptom frequency and duration differently
Dopamine agonist performs in patients with early stage and advanced disease
Early assessment could affect clinical decision making
Systems genetics approach sets stage for lab testing of simvastatin and other candidate drugs
Study aims to inform an enhanced approach to exercise as medicine
New tool for general neurologists aims to streamline differential diagnosis
When and how a multidisciplinary palliative care clinic can fill unmet needs for this population
Randomized trial demonstrates noninferiority to therapist-led training