Singular research aims to enhance prediction of rupture
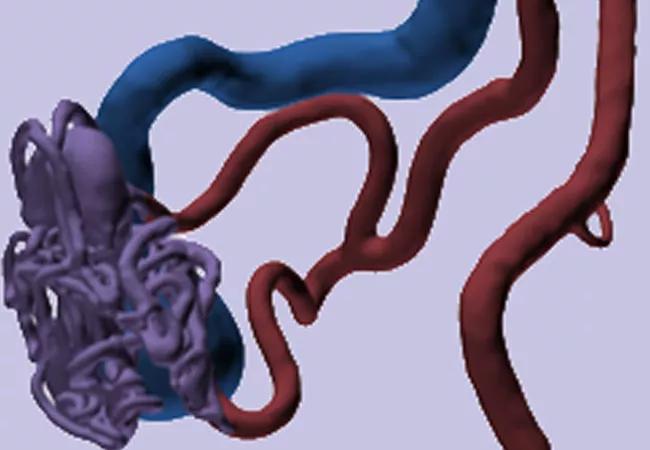
A Cleveland Clinic research team is developing a quantitative model that can characterize the integrity of an arteriovenous malformation (AVM) based on fluid-structure interactions to more accurately evaluate rupture risk.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Determining whether a blood vessel can withstand the flow of blood is key to predicting whether an aneurysm or AVM is likely to rupture. At present, however, predicting such an event — and deciding whether to recommend a potentially risky intervention to prevent it — must be based mostly on evidence from retrospective studies and a neurosurgeon’s own experience.
“In cerebrovascular neurosurgery, each patient evaluation involves perilous decisions,” says the research team leader, Nina Moore, MD, a Cleveland Clinic neurosurgeon with a background in biomedical engineering. “There is a critical need for a better evaluation strategy based on a patient’s unique anatomy.”
AVMs are particularly risky from an engineering perspective, as they consist of abnormally high-flow artery-to-vein connections, explains Dr. Moore. And hemorrhage often has dire clinical consequences: 20% of initial survivors die in the first three months, and about 45% of the remaining survivors are left with severe disability, with another 30% sustaining mild to moderate neurologic deficits.
Certain qualitative features can help classify an AVM as higher-risk, such as the presence of an intranidal aneurysm, a prior hemorrhage, venous outlet stenosis, deep location, sole deep venous drainage, small size and associated flow-related aneurysms.
But even after estimating risk based on these factors, management decisions can be difficult, compounding uncertainty about true risk. Medical management alone with blood pressure control, high-risk behavior reduction and routine surveillance may not be adequate to prevent an event. At the same time, intervention — whether with resection, radiation, embolization or a combination of these strategies — entails potential hazards of its own.
Advertisement
“The high stakes and uncertainty make having a risk-prediction model based on more quantitative factors all the more important,” says Dr. Moore.
The pressure of blood flow and the integrity of the blood vessel wall are the key factors in determining whether a hemorrhage will occur. Dr. Moore likens fluid-structure interaction modeling to trying to determine if a water balloon will burst: one needs to know not only the water pressure but also the strength of the balloon itself.
She says that while other investigators are focusing on determining AVM risk based on fluid dynamics, her team is the only group she knows of that is also incorporating blood vessel structural characteristics into a model.
Dr. Moore’s laboratory has four primary aims, she explains.
1) Understand the histologic and mechanical structures of cerebrovascular tissue. Using human cadaver heads, the lab’s investigators are focusing on histologic properties of cerebral arteries and veins by measuring elastin thickness relative to overall vessel thickness and diameter. Findings will be compared with data from the International Study of Unruptured Intracranial Aneurysms, which serves as an atlas for fluid-structure interaction modeling parameters for vessel thickness.
The team is also designing a mechanical testing rig to assess the viscoelasticity of cerebral arteries and veins. A pilot rig has been developed that uses a laser device to measure distension of vessels with pressures within the normal physiologic range. This will enable expansion of current modeling of cerebral vasculature to accurate structural data for fluid-structure interaction modeling.
Advertisement
2) Understand the fluid flow properties of cerebral AVMs. Fluid flow and pressure monitoring is being evaluated with a simple AVM simulated with 3D printing software (Figure). The system is in line with a cardiac pump to simulate blood flow through the normal range of blood pressures, with pressure and flow probes connected at different parts of the 3D-printed AVM model. A port of injection is included in the flow circuit to allow for angiographic injectors to be connected and thereby test the increase in flow through the circuit.
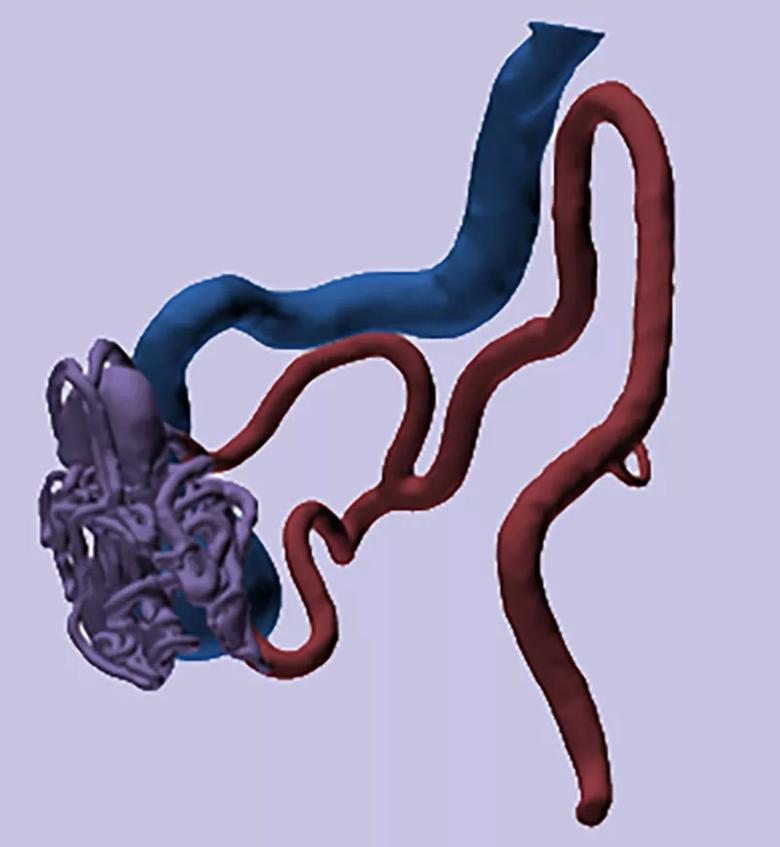
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7c7b7ea2-8b14-4c20-91e3-bacc03ed78b5/20-NEU-2021313-Dr_-Moore_CQD-insets_805x874_jpg)
Figure. Stereolithography rendering of a simple AVM 3D-printed flow model.
3) Incorporate fluid flow and mechanical structure properties of vessels into a fluid-structure interaction model. Data from the first two aims will be combined to create a fluid-structure interaction model to be used initially for simple, and then increasingly complex, AVMs. “A model of this type will enable us to preoperatively predict rupture patterns within cerebral AVMs,” Dr. Moore explains. “It will also help neurosurgeons develop better strategies for AVM intervention by modeling the effects of tying off different vessels so that we can more safely determine which should be eliminated first.”
4) Determine patient AVM anatomy via imaging. The research team is mapping out AVM nidus anatomy by comparing results from different imaging modalities and examining en bloc resections. Intraoperative measurements, taken with a transonic probe, are used in 3D renderings for flow model evaluation.
Advertisement
“Our overarching goal is to have a validated model in about five years,” says Dr. Moore. “We expect it to offer a leap forward for evaluating and predicting AVM behavior.”
Advertisement
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help