Perspectives and learnings from our Heart, Vascular & Thoracic Institute

Cleveland Clinic’s Miller Family Heart, Vascular & Thoracic Institute (HVTI) was actively recruiting patients for at least 75 clinical trials when COVID-19 arrived in the U.S. Institution-wide changes made to protect patients, clinicians and research staff from unnecessary risk triggered a cascade of events impacting every aspect of clinical trial operations. Most trials came to a halt while physicians and research administrators identified obstacles and implemented solutions that would allow studies to resume as quickly as possible.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We had to find ways to maintain the integrity of our clinical research program and jump-start the trials, which was a challenge in the presence of so many restrictions,” says Samir Kapadia, MD, Chairman of Cardiovascular Medicine at Cleveland Clinic.
On March 18, Cleveland Clinic halted nonessential procedures and nonurgent outpatient appointments. This meant the lion’s share of clinical trial participants could no longer be seen in person. Clinical research staff were faced with developing alternative methods for screening, consenting, treating and evaluating hundreds of patients.
“We went from in-person to virtual visits overnight,” says Denise Kosty Sweeney, MSN, RN, Research Administrator for HVTI. “It was a challenge figuring out how to consent patients virtually and teach them how to use the technology.”
Virtual visits were sufficient for evaluating some clinical trial participants, but not all. To gather requisite data that could not be obtained virtually, Cleveland Clinic enlisted clinicians to visit patients’ homes, where they conducted physical exams and echocardiograms and obtained blood for lab testing.
Distribution of study drugs was a major hurdle. Medications could be delivered to in-state patients via Cleveland Clinic’s pharmacy services, but arrangements had to be made for study sponsors to ship medications to participants outside Ohio.
In mid-April, sponsors formally suspended some 60% of cardiovascular trials that Cleveland Clinic was involved in. “At this point, we went through our studies and identified the ones that could continue,” says Kosty Sweeney. These included studies providing new treatment options for patients undergoing essential procedures, including aortic valve replacement and nonelective stenting.
Advertisement
While protocols to accommodate Cleveland Clinic patients were being revised, adjusting multisite national and international trials posed a different set of problems for the Cleveland Clinic Coordinating Center for Clinical Research (C5Research). As one of the nation’s leading academic research organizations in the cardiovascular field, C5Research designs, plans and manages large multicenter trials.
Sites were impacted by COVID-19 to varying degrees, according to A. Michael Lincoff, MD. “The national site coordinators within each country have been reaching out to struggling sites to help with local pandemic-related issues,” says Dr. Lincoff, who also serves as Vice Chair for Clinical Research in Cleveland Clinic’s Department of Cardiovascular Medicine. “We arrange for them to have regular discussions with trial sponsors about how to overcome these issues.”
For clinical trials still in the early stage, virtual meetings with site coordinators and staff were substituted for in-person visits. However, the virtual platforms adequate for small group discussions proved problematic for large meetings. “We had to find a new platform that provided high-quality video and audio, could accommodate large meetings and had good connectivity with overseas sites,” explains Ruth Cannata, BSN, RN, Director of Operations for C5Research.
While changes in protocol were designed to keep as many clinical trials as possible on track, the ultimate goal was to return to normal operations when Cleveland Clinic resumed elective procedures and nonurgent outpatient visits. For patient safety, this was undertaken in stages.
Advertisement
“We analyzed our trials to see what it would take to minimize patient exposure to COVID-19, and we decided to start with low-risk studies,” explains Dr. Kapadia. “These involve patients who don’t need to travel or undergo any testing other than what is considered standard of care.” These low-risk trials resumed in early June.
In mid-June, trial-related visits resumed for study participants who were undergoing testing solely for research, not as part of routine care. As of mid-July, healthy individuals can be enrolled as control subjects in a trial if they are present at a Cleveland Clinic facility for other reasons (e.g., as a Cleveland Clinic employee, a student or a family member accompanying a patient).
Resuming multicenter trials administered through C5 Research has been more complex. “We are modifying protocols as necessary, reactivating sites globally and enrolling new sites as they are permitted,” says Dr. Lincoff.
As clinical trial activity ratchets up under new conditions, one overarching concern remains: How will outcomes and data interpretation be affected?
“There is tremendous potential for missing and distorted data,” Dr. Lincoff cautions. “Patients who die because they are afraid to go to the hospital may be lost to follow-up, or the cause of death may end up being unobtainable. For instance, if a patient died of COVID-19 and had elevated troponin levels, can we know whether or not they had a myocardial infarction?”
“Losing patients to follow-up would have a huge impact on the credibility of a study and the interpretation of data,” adds Dr. Kapadia. “This is why our investigators pursue every contact and every avenue to achieve 100% follow-up.”
Advertisement
That aggressive approach is paying off. During the pandemic, C5Research wrapped up the 13,000-patient international STRENGTH trial in patients with mixed dyslipidemia, obtaining vital statistics in 99.8% of participants and follow-up in 96.6%.
“I’m proud of what we were able to achieve,” says HVTI Chief Academic Officer Steven Nissen, MD, who served as study chairman for the STRENGTH trial.
Reduced patient activity during the early weeks of the pandemic had one silver lining: It gave HVTI staff more time to do research. When Cleveland Clinic’s institutional review board (IRB) announced it would prioritize research projects related to COVID-19, ideas poured in at an unprecedented rate.
“The Office of Sponsored Research said, ‘Get the trials done. We’ll worry about funding later,’” notes Dr. Lincoff.
As a result, 42 COVID-19-related studies by HVTI staff have been added since March, including five prospective interventional trials.
Despite the stress of reorganizing trial processes overnight, some of the changes instituted during the pandemic have improved the way clinical trials are run. “Like everyone else, we had some processes that were outdated, but there was inertia against change because clinical trials are so highly regulated,” Dr. Lincoff says. “COVID-19 forced us to make changes with the blessing and encouragement of the FDA.”
For instance, virtual visits are likely to be retained for clinical trial participants. “They make better use of physicians’ and coordinators’ time,” Dr. Lincoff says. “Patients don’t have to travel, and it’s easier to keep them engaged. Interactions between sponsors and sites are more efficient as well.”
Advertisement
While there is no lack of enthusiasm for research in Cleveland Clinic’s HVTI, the IRB has gone to great lengths to keep up. Before any trial can resume, an application to restart must be submitted and reviewed. This has required committee members and staff of the IRB to meet two or three times a week.
“Every week we evaluate more trials,” says Dr. Kapadia. “We’re fired up about getting them resumed and initiating new ones.”
Advertisement
Patients report improved sense of smell and taste
Clinicians who are accustomed to uncertainty can do well by patients

Unique skin changes can occur after infection or vaccine

Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition

As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients
Early results suggest positive outcomes from COVID-19 PrEP treatment
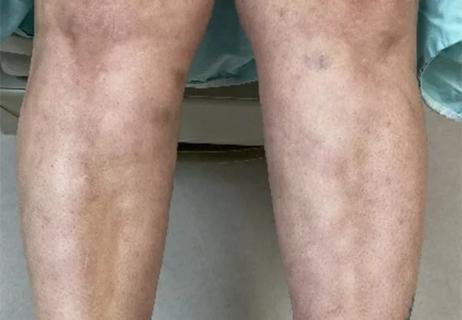
Could the virus have caused the condition or triggered previously undiagnosed disease?

Five categories of cutaneous abnormalities are associated with COVID-19