Anti-viral immunity identified a novel mechanism in necroptosis
Researchers at Cleveland Clinic’s Center for Pathogen & Human Health Research have identified a novel mechanism for how the body defends itself against viruses using programmed cell destruction.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A study published in Nature Cell Biology identified a new role for the protein OASL in necroptosis, where cells self-destruct to prevent viruses from surviving and replicating. Necroptosis controls viral replication and causes inflammation, putting the body into a state of strong and efficient defense to clear the virus, said Shin Ae Lee, PhD, first author on the paper and a postdoctoral fellow in Cancer Biology.
Mapping out the mechanisms behind defense strategies like necroptosis supports future studies into host-pathogen standoff, a tug-of-war in the immune system during virus infection. Research into these detailed pathways is essential for understanding how viruses infect and survive in humans, and can translate into treatments or vaccines.
Numerous viruses are known to trigger necroptosis, including influenza A virus, cytomegalovirus and herpes simplex virus-1, a widespread virus estimated to effect as much as 80% of the population. Immune responses like necroptosis prevent prolonged infection. Viruses spending more time in the body can cause chronic symptoms and long-term damage, as the body sustains inflammation to fight off the virus.
Dr. Lee works in a lab led by Jae Jung, PhD, chair of the Cancer Biology Department, director of the Infection Biology program, and director of the Global Center for Pathogen & Human Health Research. The Jung Lab studies virus-induced cancers and infectious diseases and how pathogens spread and cause disease.
Necroptosis was recently identified as a critical anti-viral innate immune response, but mechanistic details were unclear. This study found a novel regulator of necroptosis, OASL, acts as a “chaperone” for building the necrosome, a protein complex that mediates cell death. OASL forms phase-separated liquid droplets that attract the molecular components necessary to induce necroptosis, providing a platform for these interactions to take place.
Advertisement
Part of Dr. Lee’s research is studying how some viruses, such as vaccinia virus, evade necroptosis. Vaccinia is in the same family as the virus that causes smallpox, and is used to vaccinate against smallpox. Some viruses can knock out, or even mimic, proteins in host cells that disable defenses like necroptosis.
Researchers still don’t know the details of what allows vaccinia to evade necroptosis, but understanding the necroptosis pathway supports further investigation.
“This viral immune evasion mechanism may provide insights into how other viruses utilize a similar method to evade the anti-viral necroptosis pathway,” Dr. Lee says. “For example, the current emerging virus, Mpox, belongs to the same family of viruses as vaccinia virus, and may utilize similar methods for host immune evasion.”
Mpox, formerly known as monkeypox, caused nearly 30,000 U.S. cases and 20 deaths in 2022, according to the Centers for Disease Control and Prevention.
Necroptosis is associated with many human diseases with inflammatory processes outside of viruses, including cancer, sepsis, neurodegenerative diseases and atherosclerosis. Further research may also show OASL-mediated necroptosis is involved in these processes, Dr. Lee says.
This work was partly supported by NIH grant numbers CA200422, CA251275, AI140718, AI140705, AI140705S1, AI152190, AI171443, AI171201, DE023926, DE028521 and KGM9942011.
Editor’s Note: This article was originally published by Cleveland Clinic Lerner Research Institute.
Advertisement
Advertisement

Not if they meet at least one criterion for presumptive evidence of immunity

Findings challenge the current diagnostic criteria for congenital Zika syndrome, expanding the definition beyond skull or brain abnormalities

Chemicals in e-cigarettes weaken the epithelial barrier in airways
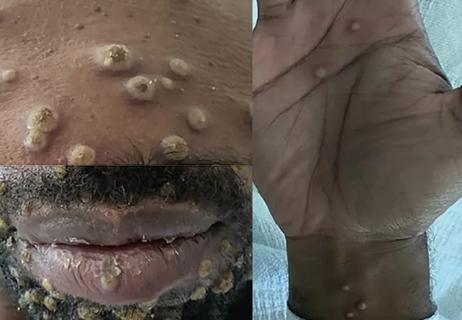
Persistent rectal pain leads to diffuse pustules

Use of MRI adds to cost but remains cost-effective due to higher sensitivity

Collaboration must cross borders and disciplines

Investigating asymptomatic parasitemia will also contribute to knowledge of disease immunity
How our Advisory Services and Affiliate Program has helped hospitals drive down SSI rates