Inhibiting gene expression boosts treatment
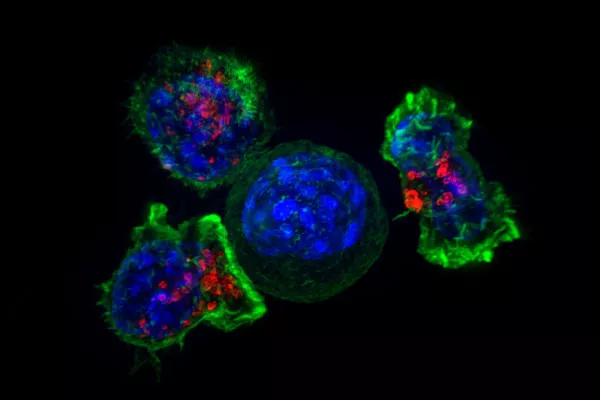
The use of chimeric antigen receptors (CAR) to enhance the ability of the immune system’s T cells to target and kill cancer cells has advanced the treatment of patients with B-cell malignancies. However, CAR T-cell therapy for solid tumors has lagged, due to the complex microenvironment of solid tumors that includes suppressive cells, T-cell-intrinsic negative regulatory mechanisms and overexpression of inhibitory molecules.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Recently, Cleveland Clinic researchers studied whether inhibiting the gene indoleamine-pyrrole 2,3-dioxygenase (IDO1), which is over expressed in many cancers, along with using CAR T-cell therapy might prove to be an effective combination treatment for patients with solid tumors — specifically colon cancer. Their work was published in the Journal of Hematology and Oncology.
One way that tumor cells evade immunosurveillance is the upregulation of the IDO1 enzyme that catalyzes tryptophan degradation in the kynurenine pathway. Tryptophan depletion and its metabolite kynurenine create an immunosuppressive tumor microenvironment.
“We targeted the tumor microenvironment by downregulating IDO1 which allowed the CAR T to penetrate tumors and enhance the cancer therapy,” says Yong Li, PhD, cancer biology, Cleveland Clinic, and senior author on the paper.
Dr. Li and his colleagues used human cancer specimens and cell lines to test their hypothesis. They began by analyzing IDO1 expression in tumors from 382 patients with colorectal cancer and found that the average overall survival was reduced by five months in patients with high IDO1 expression compared with those with low IDO1 expression.
From there they studied the regulation of IDO1 gene expression mediated by microRNAs. Using the computational program TargetScan, which predicts biological targets of microRNAs, they found that miR-153 and nine other microRNAs putatively target the IDO1 gene. Next, they revealed that only miR-153 is an authentic inhibitor of IDO1 using reporter gene expression assays.
Advertisement
Later they transfected miR-153 mimics or a negative control into colon cancer cells lines and treated the cells with the cytokine interferon gamma, IFN-γ. Compared with mock treatment or a negative control, the miR-153 mimics substantially decreased IFN-γ-induced IDO1 expression in all tested cell lines as determined by flow cytometry and by 60-90 percent by western blotting. Finally, they corroborated the results using several other methods.
“These data suggested that IDO1 is a bona fide target gene of miR-153,” says Dr. Li.
The researchers also performed a series of in vitro cell-based assays finding that cell proliferation, apoptosis and cell cycle arrest were not changed by miR-153 overexpression in DLD-1 and HCT-116 cells with or without IFN-γ treatment. There was little to no alteration of cell proliferation, cell migration and colony formation when miR-153 was introduced to the two cell lines.
“Previous studies have shown that miR-153 overexpression reduces cell proliferation in lung cancer cells, melanoma cells and glioblastoma stem cells but not in colon cancer cells,” says Dr. Li. “Our data supports this and suggest that miR-153-mediated cell proliferation regulation is cancer cell-type specific.”
Dr Li says even though the miR-153 downregulated IDO1 expression about 50 percent in HCT-116 and DLD-1 colon cancer cells, it did not affect cell proliferation, cell migration and colony formation of these cells. This moderate downregulation of IDO1 had little effect on the cancer cells, he says.
Advertisement
The researchers constructed a CAR against colon cancer cells using EGFR as a target. They used a “placeholder” CAR that is designed to target an EGFR variant found specifically in cancer (EGFRvIII), yet has restrained activity for EGFR, and tested it in immunodeficient mice.
“We found the CAR T cells killed colon cancer cells with miR-153 overexpression but were less effective against colon cancer cells without miR-153 overexpression,” Dr. Li says. “There was a survivor advantage for the mice who received the microRNA and the CAR T-cell therapy.”
He says the next step will be to develop microRNA mimics that can be used directly in a patient at the same time as CAR T-cell therapy to see if the combination works better than CAR T alone in human subjects.
Image source: NIH Image Gallery under license https://creativecommons.org/licenses/by-nc/2.0/.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology