New reviews offer insights on immunotherapies
There are gaping holes in our knowledge of the pathobiology of SARS-COV-2 as it interacts with our immune defenses, according to Leonard Calabrese, DO, Director of the R.J. Fasenmyer Center for Clinical Immunology at Cleveland Clinic. As the medical community struggles to understand and limit the impact of the rapidly evolving COVID-19 pandemic, there are more questions than answers.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Cleveland Clinic rheumatologists set out to address some of these issues for physicians in a recently published series of reviews. The short reviews, written by Cassandra Calabrese, DO, Leonard Calabrese, DO, and Emily Littlejohn, DO, MPH, and collaborators, appear in Cleveland Clinic Journal of Medicine’s collection of COVID-19 Curbside Consults.
Dr. Leonard Calabrese discusses a hypercytokinemic inflammation syndrome in patients with advancing COVID-19. Also referred to as a cytokine storm, the syndrome primarily targets the lungs, leading to acute respiratory distress syndrome (ARDS), which shares some clinical and pathologic symptoms with idiopathic ARDS.
“Laboratory features are quite similar among these disorders, with marked elevations of acute phase reactants (i.e., C-reactive protein, ferritin), lymphopenia, coagulation defects and elevated levels of numerous inflammatory cytokines; prominent among them are interleukins 6 (IL-6), 1 (IL-1) , 2 (IL-2), 7 (IL-7), and 17 (IL-17), granulocyte macrophage-colony stimulating factor (GM-CSF) and tumor necrosis factor (TNF),” Dr. Leonard Calabrese writes.
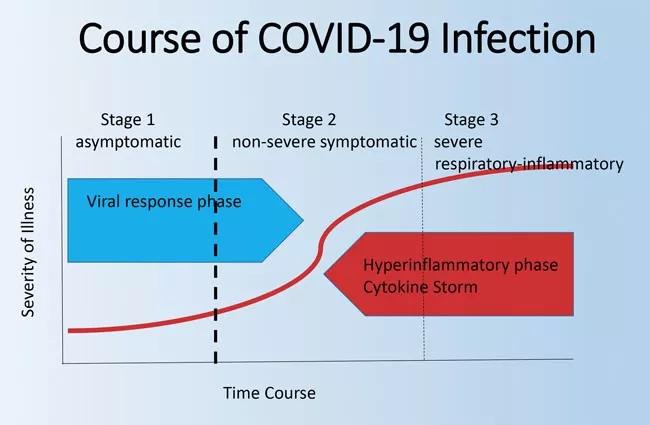
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7cb5e6e3-e4da-450d-8f3d-6c113f1ab196/rheum_jpg)
To read the review in its entirety, along with complete references, click here.
In a separate review article, Drs. Cassandra Calabrese and Leonard Calabrese, along with critical care specialist Prabalini Rajendram, MD, and clinical pharmacist Gretchen Sacha, PharmD, discuss practical the aspects of targeting IL-6 in the treatment of COVID-19. The physicians discuss currently available IL-6 inhibitors, including the mechanisms of action, current dosing recommendations and contraindications for tocilizumab, sarilumab and siltuximab. Importantly, the group notes that the “Centers for Disease Control and Prevention makes no recommendations for the use of any specific agent for treating COVID-19, since no agent has regulatory approval.”
Advertisement
The authors warn that although “anti-IL-6 drugs are being widely used experimentally and as off-label therapy for patients with COVID-19 who are sick and deteriorating but have a reasonable chance of recovering, [] they are still unproven and unapproved for this use.” The entire review, along with complete references, is available here.
With multiple potential anti-viral mechanisms of action and immunomodulatory effects through attenuation of cytokine production, hydroxychloroquine (HCQ) is another potential experimental treatment for COVID-19. In her review, Dr. Littlejohn discusses the potential risks and available clinical data regarding the use of HCQ. Importantly, she writes: “There are no clear data indicating that HCQ has a favorable effect on outcomes in COVID-19.” Dr. Litllejohn’s complete discussion of HCQ for COVID-19, along with her reference list, is available here.
Due to shortages of HCQ, frequently used in the treatment of systemic lupus erythematosus, rheumatoid arthritis and other immune-mediated diseases, patients may have difficulty filling prescriptions, Dr. Cassandra Calabrese says.
“In these scenarios, and given the long terminal half-life of the drug, I have been recommending that patients, based on their disease and dependency on the drug, to decrease their daily dose by half, to extend the supply of their current prescription; we feel this is safe for short periods (1–2 months or more),” Dr. Calabrese writes. “This would be more appropriate for rheumatoid arthritis and or mild systemic lupus erythematosus, and less appropriate for lupus patients with significant end-organ involvement. In my opinion, this is an imperfect solution but a reasonable compromise. Hopefully, drug availability will not be an ongoing issue as production is being ramped up and regulatory steps are being taken to protect our patients, albeit in limited fashion.” To read more from Dr. Calabrese on COVID-19 and your rheumatology patients, along with a complete reference list, click here.
Advertisement
Data on treatment of COVID-19 are rapidly evolving. To date, there is no evidence from randomized controlled trials that any single therapy improves outcomes in patients infected with COVID-19. There are more than 270 registered clinical trials (as of April 13, 2020), and a number of agents have been proposed or are in use, including anti-IL-1, anti-GM-CSF, anti-TNF and Janus kinase inhibitors. Among these therapies, agents targeting IL-6 have generated the greatest enthusiasm, according to Dr. Leonard Calabrese.
In response to the pandemic, the rheumatology community has formed the COVID-19 Global Rheum Alliance, which Dr. Cassandra Calabrese describes as “an international effort with a goal to aid clinicians and researchers in real time as the pandemic evolves, providing insights into how COVID-19 is impacting patients with rheumatic disease…The registry should provide further insight in to the impact of COVID-19 on this vulnerable patient population.”
Advertisement
Advertisement

Patients report improved sense of smell and taste

Clinicians who are accustomed to uncertainty can do well by patients

Unique skin changes can occur after infection or vaccine

Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition

As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients

Early results suggest positive outcomes from COVID-19 PrEP treatment

Could the virus have caused the condition or triggered previously undiagnosed disease?

Five categories of cutaneous abnormalities are associated with COVID-19