Researchers testing innovative design
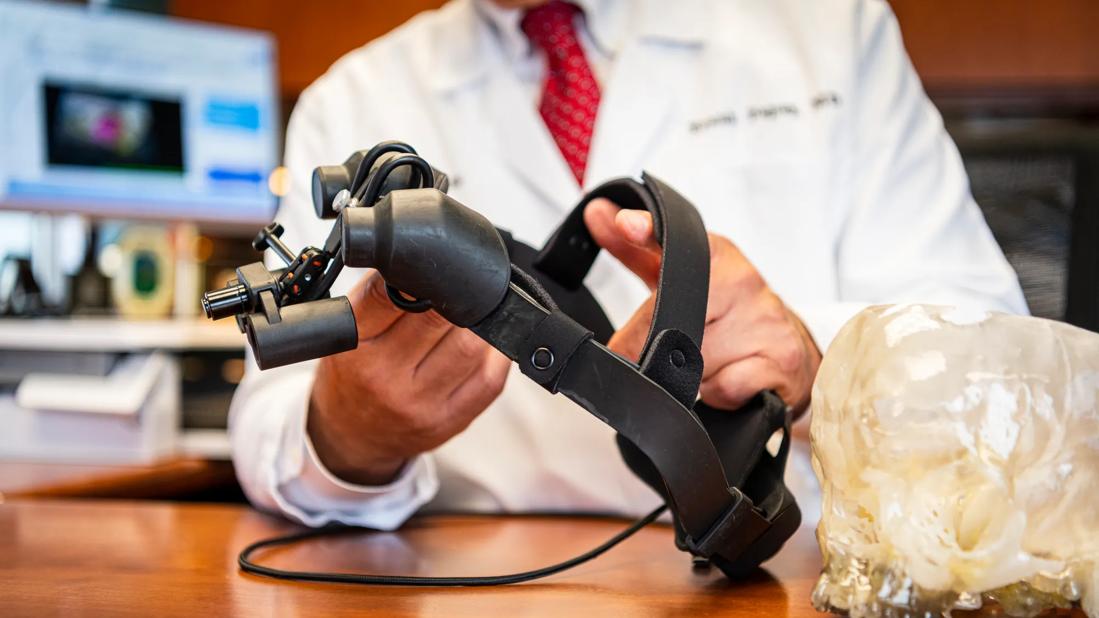
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/664b0540-3016-4344-8921-c290ded90744/PHI_2993424ab_09-22-22_046_SG-jpg)
googles_650x450
Physicians at Cleveland Clinic are investigating the use of newly developed prototype goggles to perform sentinel node biopsies in women with breast cancer. SmartGoggles is a real-time fluorescent imaging and display system that captures the disease in 3-D and presents it to the surgeon. Francis Papay, MD, Chair of Cleveland Clinic’s Dermatology and Plastic Surgery Institute, who also has a degree in biomedical engineering, worked closely on their development with SmartGoggles inventor Yang Liu, Ph.D, at the University of Akron.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The use of the goggles is much like the loops or magnifiers that surgeons have been wearing for years,” says Dr. Papay. “Instead of a magnifier, a surgeon would wear an electronic device that uses a kind of augmented reality to allow visualization of several layered pictures of the patient in real time.”
“SmartGoggles provide surgeons a better way to guide surgery and visualize disease,” says Dr. Liu. “They have the capability to find where a tumor is and display it to the surgeon.” SmartGoggles can detect fluorescent objects with as low as 60 nM indocyanine green (ICG) and can distinguish structures down to 0.25 mm with large-field-of-view stereoscopic imaging.
A small validation study is currently underway to determine whether SmartGoggles are as effective as current standard of care methods for visualizing lymph nodes during breast cancer surgery. “One of the challenges with the current method of doing sentinel node biopsies is that it can sometimes be difficult to correlate the surgical landscape with what is seen in imaging,” says Stephen Grobmyer, MD, Director of Breast Cancer Surgery at Cleveland Clinic Cancer Center and the study leader. “SmartGoggles allow the surgeon to more accurately see the tissue of interest.”
Traditionally, sentinel node biopsies rely on visible, non-fluorescent dyes. This means surgeons must sometimes perform a lot of dissection to locate the sentinel nodes. With SmartGoggles, surgeons can see both fluorescent and visible light, allowing for a more accurate determination of the depth of the nodes. If fluorescent dye is used in surgery, the surgeon is required to hold and manipulate a camera while operating. The goggles eliminate that problem by allowing the surgeon to directly visualize the fluorescent dye.
Advertisement
The study will utilize the SmartGoggles to visualize sentinel lymph nodes in six patients who have been diagnosed with breast cancer. “We will compare the fluorescence-guided surgery using SmartGoggles with the current standard of care using radiocolloid and/or blue dye,” explains Dr. Grobmyer. “We will then study and confirm that the use of the goggles with fluorescent dye identifies the same lymph nodes as the traditional mapping techniques.” Depending on the initial results, the procedure may be performed on additional patients.
“Based on previous studies using fluorescent dye with cameras, we anticipate that this will be feasible and will identify the same nodes,” says Dr. Grobmyer. The study will also include an interpretation of data from the histological examination of the tissue.
In the future, the goggles may be used to assist surgeons in removing tumors and determining whether clean margins have been achieved. Another application for the goggles is in the developing field of multispectral imaging. Using the goggles for multispectral imaging would complement new advances in the creation of tags and dyes that are able to target different types of tissue.
“We are excited about the goggles, not just for breast cancer patients, but for patients with many types of tumors,” says Dr. Papay. “This is the first stage of the future of surgical navigation, enabling us to target tumor types and utilize an augmented reality instrument. It is a groundbreaking way of looking at tumors.”
Advertisement
Advertisement
Clinical trials and de-escalation strategies
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality