Novel prosthesis aims to fill an unmet clinical need
Newborns with a congenital mitral valve defect — e.g., parachute mitral valve, mitral arcade, double-orifice mitral valve or Shone’s complex — currently have no optimal treatment options. The smallest commercially available mitral valve prosthesis is 15 mm. There are no prostheses for infants, in whom the mitral valve diameter is sometimes as small as 10 mm. Moreover, currently available prostheses aren’t expected to last longer than five years, requiring multiple replacements as a child grows.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Repair in infancy is often just palliative until the infant grows to accept a large prosthetic valve,” observes Joanna Ghobrial, MD, a cardiologist with Cleveland Clinic’s Adult Congenital Heart Disease Center. “The defect can lead to irreversible detrimental cardiac remodeling, eventually resulting in morbidities in adulthood such as propensity for atrial arrhythmias, pulmonary hypertension and decreased cardiac output.”
Affected patients often present as adults who have had several open-heart surgeries for mitral valve replacements, in addition to long-term complications including atrial arrhythmias and thromboembolism. “This puts them at overall higher risk for redo cardiac surgery,” Dr. Ghobrial adds, “thereby increasing morbidity and mortality in an already vulnerable population.”
“It’s an unmet need,” says Hani Najm, MD, Chair of Pediatric and Congenital Heart Surgery at Cleveland Clinic. “We don’t have a prosthesis that will fit the small newborn mitral valve.”
The problem is one that Dr. Najm has been pondering for years, ultimately spurring him to lead a Cleveland Clinic team that has developed a prototype stented pulmonary autograft prosthesis to replace the defective mitral valve. Made from a partially absorbable material that can expand and “grow” with the child, the frame of the device (shown in photo below) is designed to extend the time before a replacement prosthesis is needed.
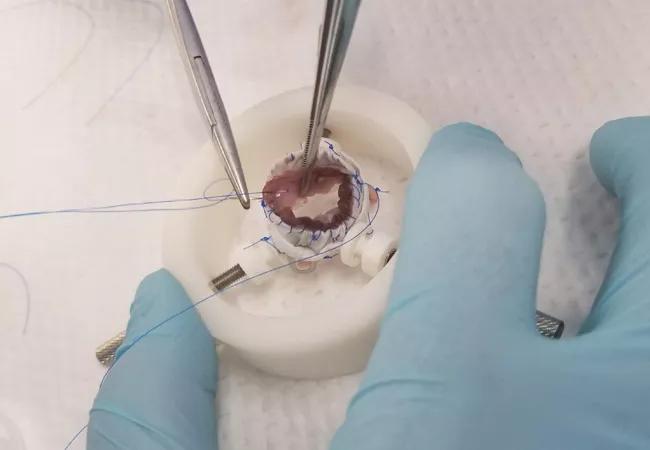
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7a7b96c5-c5a1-42cd-bdf1-a50156cbe444/19-HRT-153-Stented-Pulmonary-Autograft-CQD_jpg)
For now, the best available surgical approach for infants with these mitral valve defects is to create a Fontan circuit and simply discard the left ventricle and mitral valve. But this is far from ideal, leaving patients with less functional capacity as they grow and the probability of further surgery later in life. For females, it typically means needing to avoid pregnancy because the univentricular circulation could not support bringing a pregnancy to term.
Advertisement
The alternative prototype device developed by Dr. Najm’s team promises to address all these issues. Its use involves a modified Ross II procedure in which the patient’s own pulmonary valve is harvested and mounted into the prosthesis. The folds of the device — made from a combination of titanium and a thermoplastic polymer resin — slide over one another, allowing for prosthesis expansion. The valve encased in this scaffold is placed into the mitral valve position, as in a standard mitral valve replacement operation.
“The hope is that these children will have a valve that will grow with them,” Dr. Najm explains, “avoiding the need for multiple replacements and the need for anticoagulation.”
Once development for newborns is completed, the team hopes to use the same type of prosthesis for older patients with congenital mitral valve defects, including women of childbearing age and those with contraindications to anticoagulation.
“The development of this valve prosthesis can be quite impactful in the adult congenital population, given the potential for expansion of use in other congenital valvular defects,” notes Dr. Ghobrial.
Dr. Najm’s idea for the stented pulmonary autograft prosthesis was a major reason he chose to come to Cleveland Clinic more than three years ago, as he saw the institution as an ideal place to bring the concept to fruition. That’s thanks to the unsurpassed complexity of its case mix and the legacy of Cleveland Clinic Innovations (CCI), which has a 19-year track record of commercializing clinical products born of unmet needs identified by surgical and medical staff.
Advertisement
Working with CCI’s Senior Director of Product Development, Kelly Emerton, PhD, and a group of engineering consultants, Dr. Najm’s team has developed multiple iterations of prototypes for the prosthesis and performed feasibility testing for durability and ways to simplify the surgical procedure. Several rounds of ex vivo testing have been performed on pig hearts (obtained from a local butcher) in Cleveland Clinic preclinical research and development facilities. Further testing will begin at the benchtop as well as in adult and growing-animal validation models.
CCI is also working with the FDA to apply for Humanitarian Device Exemption (HDE) status for the prosthesis. HDE designation allows for clinical testing of devices for rare high-risk conditions to demonstrate safety with exemption from the requirement to conduct large clinical trials.
While HDE designation may come by the end of 2019, the timetable for development also depends on CCI’s success in securing funding interest.
Regardless of the timetable, Dr. Najm is grateful to finally see his idea move forward. “At Cleveland Clinic, we have the infrastructure to do it,” he says. “It may take about two to three years, but once we get this device into human use, I think it will transform how we deal with the youngest patients with mitral valve disease. It has potential to dramatically improve lives.”
Advertisement
Advertisement

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients