Locations:

Extension of DELIVER-MS trial will clarify long-term clinical effects of dueling treatment philosophies
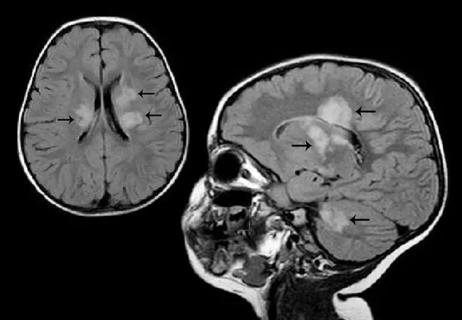
New review makes the case for earlier pediatric enrollment and other changes
Knocking out, then restoring, the immune system holds great promise

What neurologists need to know about this emerging development
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Advertisement
Advertisement