Promising new avenue to reduce pain and disability for upper extremity amputees
By Adrienne Lee, MD, FRCSC, Annika Sinha, BSc, and Peter J. Evans, MD, PhD, FRCSC
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The number of amputees in the United States (lower and upper extremity) is expected to triple by 2050.1 In 2005, nearly 41,000 people were living with upper extremity amputations, most commonly caused by high-energy trauma.2 And at any given time, nearly 20 million Americans suffer from peripheral nerve injury caused by trauma and medical disorders.3 Nerve injuries alone account for approximately $150 billion in annual healthcare costs in the U.S.4
The increasing prevalence of amputation, coupled with the fact that upper extremity amputation and nerve transection injuries can be severely disabling and painful, has led to the development of new, innovative approaches in postamputation medicine. This is especially important in the context of upper extremity amputations, which have been shown to produce an even greater degree of disability than lower extremity amputations.
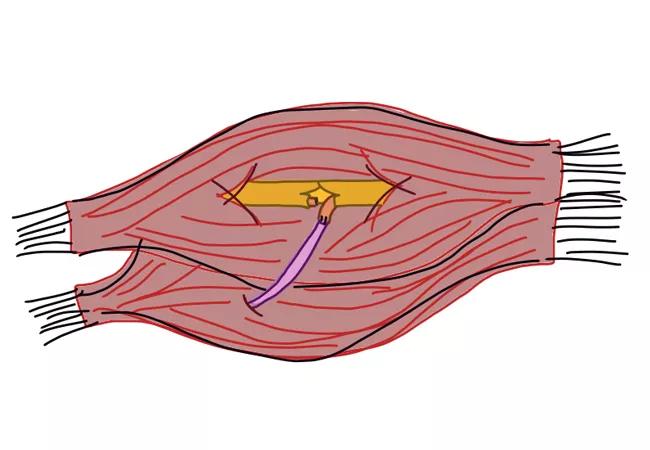
Targeted muscle reinnervation (TMR) is one such emerging technique. TMR can reduce the dysfunction and pain that upper extremity amputees experience, and help those with painful neuromas from isolated nerve injuries. A significant reduction in neuroma and phantom limb pain have been unexpected and welcome benefits of TMR.
Combined with evolving prosthetic design technology, TMR provides a promising new avenue to reduce disability for upper extremity amputees and adds to our toolbox for treating these patients.5
Kuiken and Duamanian described TMR for proximal upper extremity amputations at or above the elbow to improve myoprosthetic control in the early 2000s.6 This surgical technique uses strategic nerve transfers to either reinnervate a denervated muscle group or to create multiple points of innervation in a proximal muscle segment to aid in prosthetic control.
Advertisement
The myoelectric signaling from newly reinnervated muscle segments is picked up by transcutaneous electrodes and used to control advanced prosthetic devices. Motor control of hand and wrist function is also transmitted through these transfers because of retained neural information within the amputated nerve and its cortical connection. This allows for intuitive and enhanced control of a prosthetic limb.
Indications for TMR include:
Patients with amputations proximal to the wrist are candidates for TMR if they do not have brachial plexopathy or proximal nerve injury. In proximal, above-elbow amputations or shoulder disarticulations, the goals of TMR are to restore elbow flexion and extension and hand open and close actions.
Selection of nerve transfer patterns depends on the level of the amputation, available nerve transfers and target musculature.
For example, in transhumeral (TH) amputations, TMR is used to create hand close and hand open control signals, while preserving native innervations that perform elbow flexion and extension.7 The remnant median nerve is transferred to the short head of the biceps for hand close action and the distal radial nerve is transferred to the lateral head of the triceps for a hand open action. This process can also be modified to include wrist control. This reinnervation allows for intuitive control of four discrete motor functions (hand close and open, elbow flexion and extension), which create more natural, versatile prosthetic limb functioning.
Advertisement
Nerve transfers for forequarter amputation and TH amputation have been well described. However the optimal selection of nerve transfers for transradial amputees is still evolving along with technologic advancements of prosthetic devices. A multicenter trial is underway to further evaluate TMR as an option for treating and preventing neuroma and phantom limb pain.
The case study below illustrates how TMR can be used for treatment of neuroma pain in a below-elbow amputee, and help plan for future enhanced prosthetic fitting.
A 40-year-old female was seen in our practice for surgical consultation for management of chronic pain after a below-elbow amputation. Her limb pain, refractory to all modes of medical management, limited her ability to wear a standard prosthesis.
Her amputation stump exhibited four focal points of pain correlating to positive Tinel’s sign over the areas of pain (Figure 1).
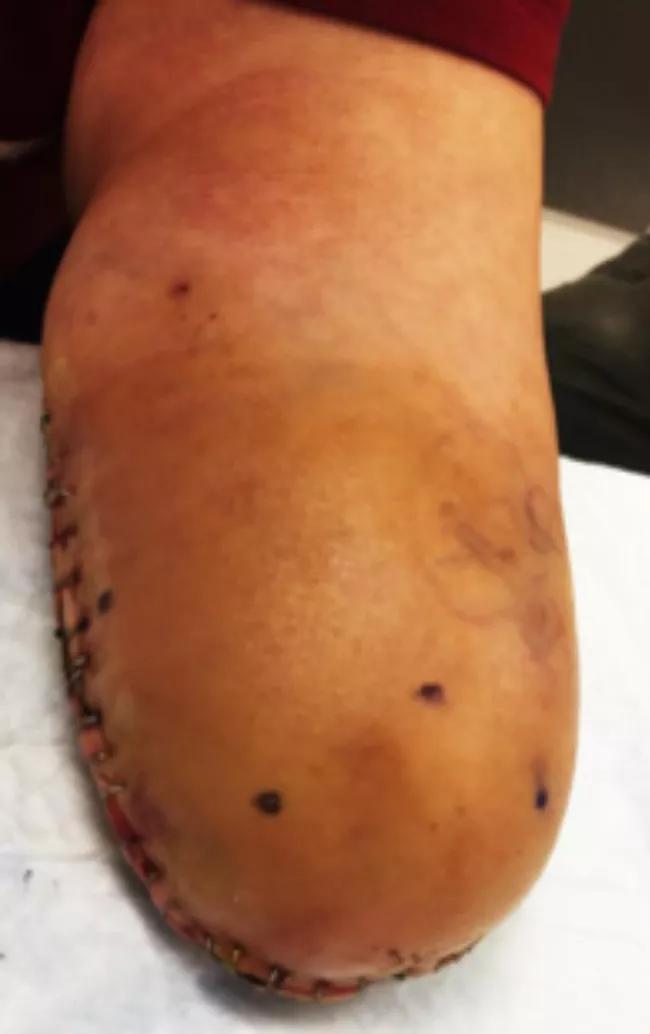
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/745f0aba-b6e9-48ed-82e1-98b16bb6eeab/18-ORT-1086-Evans-Inset-Image-01-650pxl-width_jpg)
Figure 1. Image of below-elbow stumps with marked neuromas.
After discussion with the patient and her pain management team, we decided to perform an exploration of her below-elbow stump with neuroma resection and targeted muscle transfers. We felt this had great potential to address her chronic pain and provide her with the enhanced muscle response needed to accommodate an advanced myoelectric prosthetic fitting, if she desired.
In surgery, we found neuromas of the median, ulnar, radial sensory and lateral antebrachial cutaneous nerves. All were resected until achieving visualization of healthy nerve under loupe magnification. The resected back lateral antebrachial cutaneous nerve stump was placed in a blind-ended nerve wrap and buried deep in muscle (Figure 2).
Advertisement
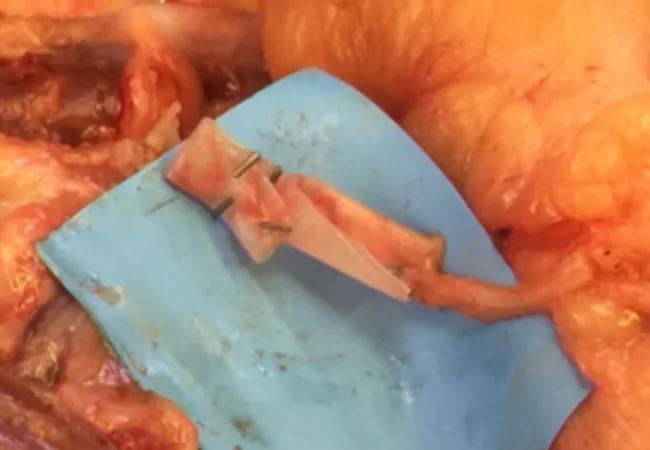
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5ee24b5f-8359-4f4f-9a09-1097a4b26a62/18-ORT-1086-Evans-Inset-Image-02-650pxl-width_jpg)
Figure 2. Intraoperative picture of blind end wrap.
We then performed three nerve transfers, each with a connector-assisted repair, consisting of two epineurial 8-0 nylon microsutures oriented at 180 degrees to each other, with fibrin glue reinforcement and a nerve protector wrapped around the repair site and detensioned.8 The following nerve transfers were performed:
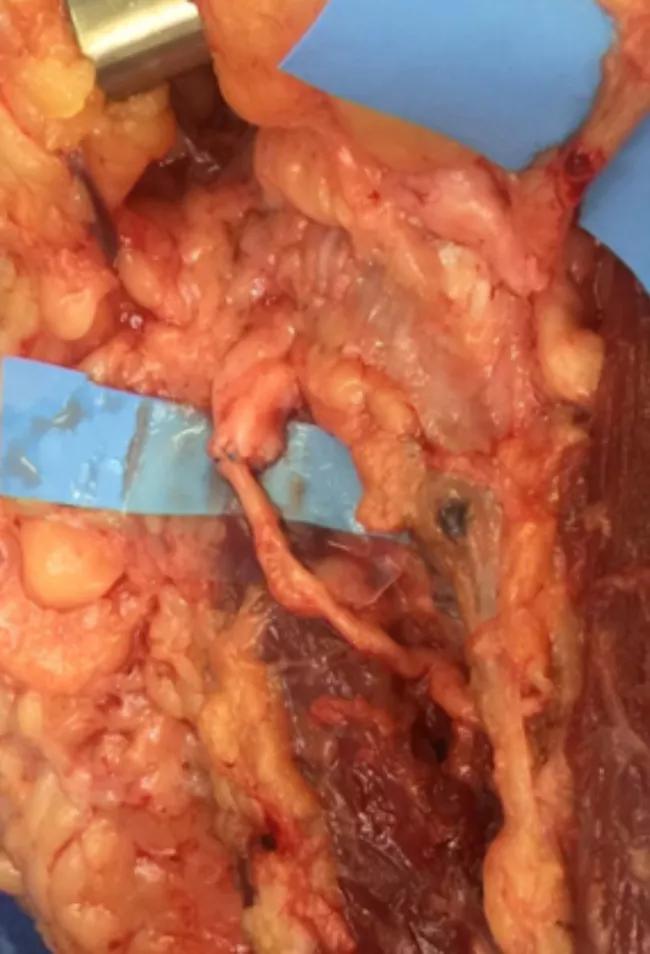
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/cf5f920f-54ee-4641-ba10-b1276235ffb7/18-ORT-1086-Evans-Inset-Image-03-650pxl-width_jpg)
Figure 3. Distal ulnar nerve to motor branch of the flexor carpi ulnaris.
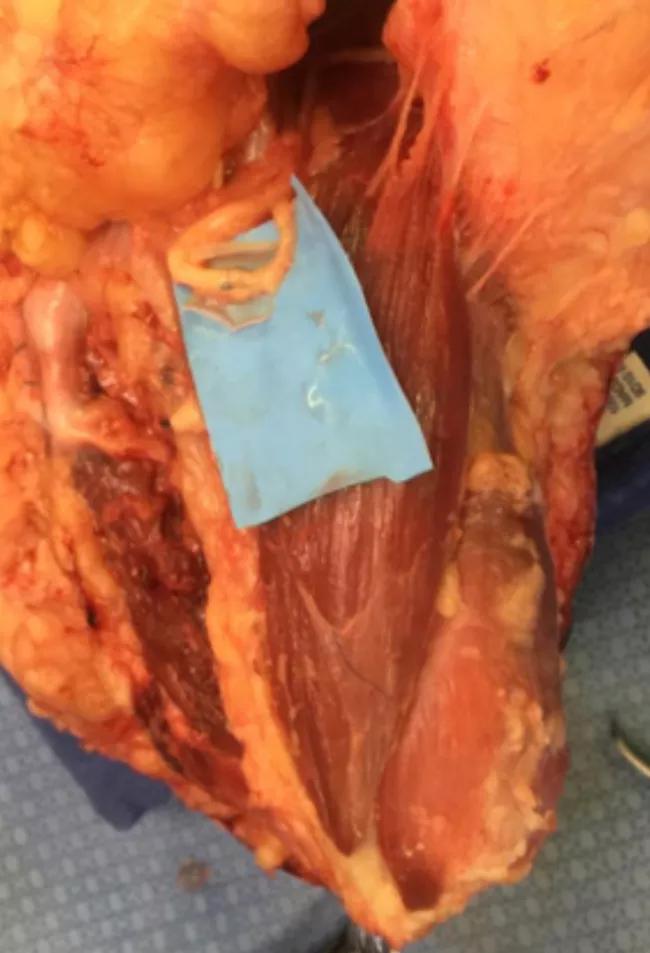
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d51acc0b-beca-48ec-b293-7f813f9f9a82/18-ORT-1086-Evans-Inset-Image-650pxl-width_04_jpg)
Figure 4. Median nerve and anterior interosseous nerve transfer to motor branches of the flexor digitorum profundus.
Postoperatively, the patient has had significant resolution of her chronic pain in her amputation stump and she is able to tolerate a prosthesis for her below-elbow amputation.
TMR is transforming our traditional treatment for patients with amputations, offering a means of reducing the incidence of neuroma formation and phantom limb pain. In addition, TMR is successful in ameliorating existing chronic neuroma pain resistant to nonoperative treatments. As our understanding improves about how TMR decreases pain, we will be able to prevent pain and suffering and reduce total cost of care for patients with amputations.
Dr. Lee is a clinical fellow in the combined hand and upper extremity fellowship at Cleveland Clinic and The Metrohealth System (Cleveland). She will be joining the department of hand and upper extremity surgery at MetroHealth after her fellowship. Ms. Sinha, a third-year medical student at Case Western Reserve University School of Medicine, is interested in pursuing a career in plastic surgery. Dr. Evans is staff in the Orthopaedic Surgery Department’s Upper Extremity Center.
Advertisement
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help