Clinical trial investigates nerve-sparing approach
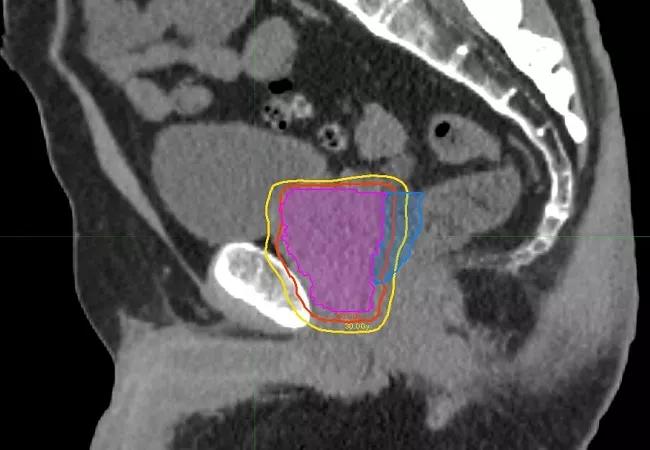
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fc68330b-6a9c-4cb6-86d2-55caf5d271af/Prostate-SAbR-650x450_jpg)
Prostate SAbR 650×450
Image-guided stereotactic ablative body radiotherapy (SAbR) has emerged as a promising up-front treatment for localized prostate cancer, with favorable biochemical response and manageable toxicities.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
However, the possibility of erectile dysfunction remains a significant concern for many patients treated for prostate cancer with either surgery or radiation therapy, including with SAbR. This has prompted efforts to find ways to harness modern technology to preserve potency by preventing neurovascular damage in erectile tissue while maintaining SAbR’s effective oncologic control.
Cleveland Clinic is among the institutions participating in a phase 2 randomized clinical trial evaluating a nerve-sparing form of SAbR in patients with early-stage prostate cancer. The Prostate Oncologic Therapy While Ensuring Neurovascular Conservation (POTEN-C) study, led by the University of Texas Southwestern Medical Center, aims to compare SAbR’s ability, with or without neurovascular sparing, to preserve erectile function while also preventing disease progression and limiting treatment-related toxicities.
“The field of radiation oncology has been very exciting in recent years because of a number of technological advancements that have improved the way we care for our patients,” says Cleveland Clinic Cancer Center radiation oncologist Rahul Tendulkar, MD, one of the co-investigators on the Cleveland Clinic arm of the POTEN-C trial. “Specifically, in the treatment of prostate cancer with SAbR, we hope to minimize the impact on patients’ daily routines and quality of life by offering treatment that can be delivered in less than two weeks and that allows patients to resume their usual activities in almost no time.”
Advertisement
Curative radiotherapy for nonmetastatic prostate cancer traditionally has been delivered slowly using conventional fractionation, either with protracted external beam treatments or permanently implanted low-dose brachytherapy seeds. More recently, moderately hypofractionated intensity-modulated radiotherapy (IMRT) has become the standard of care for delivering external radiation for localized prostate cancer.
But prostate cancer’s apparently novel radiobiology— a low “alpha/beta ratio” compared to other tumor types, creating a high sensitivity to dose per fraction — supports the concept of extreme hypofractionation in the form of SAbR as a viable alternative. In the last decade, SAbR has demonstrated several advantages in clinical practice over dose-escalated IMRT, including shorter duration of therapy (approximately two weeks vs. nine weeks) and greater convenience.
“The main advantage of using this technique is to give a tumor-killing dose of radiation in a schedule that is more convenient for patients compared to what has been done historically,” Dr. Tendulkar says.
SAbR technology has advanced to the point that extremely high doses can be safely. Typically, a dose of 40 Gray (Gy) is delivered over 5 treatment sessions, every other day, he says. “Now that there have been several thousand patients treated across the country and the world with this technique, we have stronger data that shows how effective the treatment is, and how well-tolerated the side effect profile is.”
Advertisement
The most commonly reported side effects of SAbR include increased urinary urgency and frequency, which also have been observed with conventional radiotherapy. The only published trial comparing the two treatments in early follow-up has suggested that the short-term side effect profile of both modalities is comparable.
Of special interest in the treatment of patients . is the prevention of erectile dysfunction and neurovascular damage to adjacent tissues.
The etiology of radiation-induced erectile dysfunction is not precisely known, but may involve damage to the penile neurovascular bundles, crura and/or penile bulb. SAbR’s focused beam arrangements and immobilization during treatment potentially could reduce the volume of critical structures receiving high radiation doses, thus limiting or eliminating collateral damage. The POTEN-C trial will compare standard 40-50 Gy SAbR to SAbR with dose-painting intended to preserve functionality of critical neurovascular structures on at least one side of the pelvis by reducing dosage to 30 Gy.
Dr. Tendulkar explains that approximately 30-40% of men undergoing treatment with conventional radiation therapy go on to develop treatment-induced erectile dysfunction. “It is still unclear whether SAbR will improve upon those rates,” he says. “But with this technique, we can direct the radiation dose away from those nerves that are responsible for erectile function.”
POTEN-C currently is recruiting prostate cancer patients at several high-volume cancer centers in the U.S., including Cleveland Clinic. Eligibility criteria include the presence of early-stage disease and adequate erectile function at baseline.
Advertisement
“Historically, surgeons have been able to perform nerve-sparing surgeries in an attempt to improve erectile function after prostatectomy,” says Dr. Tendulkar. “This is the first trial that is attempting nerve-sparing radiation therapy” in patients with prostate cancer.
An additional element of the trial is use of a temporary hydrogel rectal spacer, which is injected by the treating physician prior to SAbR treatment. The spacer’s purpose is to shift the position of the anterior rectum away from the prostate to provide a degree of protection by reducing radiation dose to the rectal wall. The spacer is naturally absorbed by the body a few months after treatment and usually does not typically cause side effects.
“Rectal spacers have been studied in randomized trials and confirmed to reduce the risk of rectal complications,” says Dr. Tendulkar. “They have been approved by Medicare and most insurance companies and are utilized on this clinical trial as part of standard treatment.”
Dr. Tendulkar is optimistic that the POTEN-C trial will further advance the clinical utility of SAbR already observed in clinical practice and will help improve quality of life for patients with early-stage prostate cancer.
Advertisement
Advertisement
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches