Biomarker promises a practical way to reduce misdiagnoses
There’s a strong case to be made for incorporating the central vein sign (CVS) into future versions of the McDonald criteria for diagnosis of multiple sclerosis (MS). So contends a recent Viewpoint article in JAMA Neurology by a trio of MS experts from Cleveland Clinic and Cedars-Sinai Medical Center.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The addition of the CVS to the diagnostic criteria is anticipated to provide increased specificity for the diagnosis of MS and assist clinicians in difficult cases where MRI findings cannot be conclusively determined to be supportive of MS or not,” write Daniel Ontaneda, MD, PhD, and Jeffrey Cohen, MD, both with Cleveland Clinic’s Mellen Center for Multiple Sclerosis Treatment and Research, and Pascal Sati, PhD, of Cedars-Sinai’s Department of Neurology.
These authors argue that better diagnostic tools are needed to reduce the high rate of misdiagnosis of MS, estimated at approximately 20% of cases.
The McDonald criteria specify that MS diagnosis depends on demonstration of dissemination in space (DIS) and dissemination in time (DIT) after a clinical episode characteristic of an MS exacerbation or documented progression. Changes to the criteria over time have allowed for earlier diagnosis, with resulting reductions in specificity. This reduced specificity, together with inappropriate application of MRI criteria and inadequate access to neurological care, is responsible for much of the misdiagnosis of MS, the authors note.
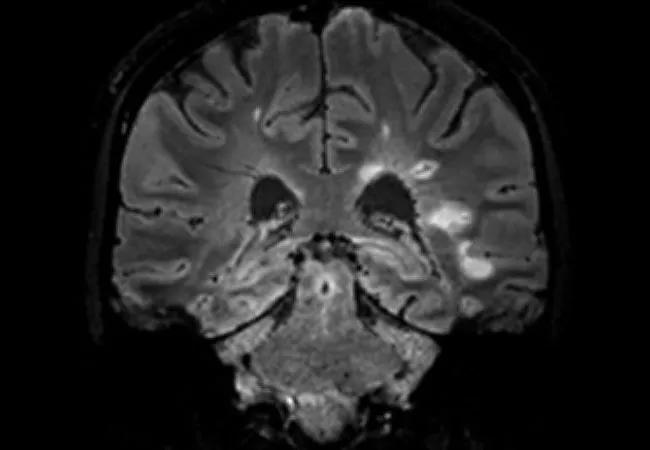
In response, they propose the CVS — i.e., a vein visualized inside a white matter lesion on T2* MRI sequences that appears as a hypointensity relative to the surrounding lesion (see example image above) — as a promising and practice-friendly candidate for helping improve MS diagnosis.
The Viewpoint article outlines a number of reasons the CVS shows much promise for real-world use as a diagnostic biomarker:
Advertisement
Despite these compelling advantages, “validation of the CVS as a diagnostic biomarker still requires some refinement,” the authors write. Several hurdles remain to be cleared:
Once these issues are addressed, addition of the CVS to the diagnostic criteria promises to bring the MS community closer to “a simpler and more straightforward diagnosis of MS,” the authors write. They note that its role will likely be refined, however, as data from the necessary prospective studies accumulate.
Advertisement
“We advocate inclusion of the CVS in the McDonald criteria as a necessary step toward a simple, stand-alone MRI characterization of MS without complex diagnostic criteria requirements or the need to demonstrate DIS and DIT,” says Dr. Ontaneda.
The Viewpoint article is available here.
Advertisement
Advertisement

Mixed results from phase 2 CALLIPER trial of novel dual-action compound

A co-author of the new recommendations shares the updates you need to know

Rebound risk is shaped by patient characteristics and mechanism of action of current DMT

First-of-kind prediction model demonstrates high consistency across internal and external validation

Real-world study also finds no significant rise in ocrelizumab-related risk with advanced age

Machine learning study associates discrete neuropsychological testing profiles with neurodegeneration

This MRI marker of inflammation can help differentiate MS from mimics early in the disease

Focuses include real-world research, expanding access and more