Study analyzes device biofilms, establishes basis for future developments
Even in the absence of infection, penile implants may harbor more — and more complex — surface-level microbiota than previously understood, according to findings from a recent Cleveland Clinic-led study. The authors report a unique microbe-metabolite interaction network from biofilms of those who are infected and asymptomatic, raising new questions about potential pathways to infection.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In the treatment of refractory erectile dysfunction, penile prostheses can be an effective surgical option. Implants have advanced considerably since first introduced in the 1950s, including developments in anti-infective irrigants and coatings to help prevent infection.
And yet device-associated infections compromise about 25% of hospital-acquired infections, according to a study published in The New England Journal of Medicine, underscoring the need for more effective preventive measures.
“Historically, it was thought that the devices were devoid of microbes in the absence of infection, but recent evidence has shown that even noninfected devices may harbor bacteria,” says first author Glenn Werneburg, MD, PhD, a urology fellow in the Department of Urology.
Co-author Scott Lundy, MD, PhD, associate staff urologist in the department, adds, “Infection is a devastating complication for 1% to 2% of men who undergo penile prosthesis implantation. The device must be removed and may not be able to replaced immediately. Afterwards, men will be unable to obtain an erection without placement of a new device.”
The research team set out to understand better the composition of the microbiota present in the biofilms of penile prosthesis devices and determine the propensity of the isolated microbes to reconstitute biofilm formation on a library of material types.
Dr. Werneburg and senior author Aaron Miller, PhD, reported initial findings from the study at the 2023 American Urological Association Meeting in Chicago, where their work was recognized with an award for Best Poster for their section.
Advertisement
Dr. Miller says the notion that clinical infections should be prevented or treated through approaches that target specific infectious processes, rather than the broad brushstroke antibiotic measures that are common today is one that resonated with the audience.
Continuing he says, “This recognition by our peers is validation that our research into the urinary tract microbiome and urologic disease is having a noticeable impact on the field.”
Dr. Miller’s lab, within Cleveland Clinic Lerner Research Institute, is one of the few laboratories in the world to focus such broad expertise on the etiology of urologic conditions. They recently led efforts to develop a global consensus on a set of standards for conducting genitourinary microbiome research.
He says this implant study—and the continued need to tackle problems presented by device-associated infections and other diseases with urinary tract microbiome origins—rely on such standards.
The research team swabbed the biofilms from patients who were scheduled for device removal or revision. Of the 27 devices that were explanted and analyzed, four were removed for infection and four for pain.
Biofilm samples and controls were subjected to a series of assays including next-generation sequencing, metabolomics and culture-based assessments to determine the bacterial composition. They also analyzed clinical associations with microbial composition.
They isolated and replicated the bacteria on various surfaces—including silicone, polytetrafluoroethylene, polyurethane, polycarbonate and titanium — in an in vivo environment designed to simulate interaction with human tissue. Explains Dr. Werneburg, “In this way, we could screen both microbes and material types for biofilm deposition.”
Advertisement
Their results show that nearly all of the devices in the study harbored robust microbiota, even in the absence of infection. Staphylococcus and Escherichia, historically linked to infection in conventional culture techniques, did not correlate with infection status. However, other uncommon genera and metabolites did, suggesting a more complex pattern at hand.
A summary of their findings are as follows:
Dr. Werneburg says the findings could lead to new information about the transition from asymptomatic to infected states and also inform the next generation of medical device coatings and materials.
“The microbe-metabolite networks present in the asymptomatic and infected devices are now being further explored to determine whether they might be conducive to or protective against infection,” he notes.
On study limitations, he acknowledges the exclusive focus on bacteria and comments, “It’s possible that other microbiota including fungi and viruses may also be important in the biofilm communities of these devices.”
Dr. Werneburg asserts, “Ultimately, we hope that the understanding generated from this study, and the methods used, may lead to the identification and development of new materials and coating for penile prostheses and other medical devices to reduce infection risk.”
Advertisement
“This study truly changes the paradigm of infections and suggests we need to work smarter, not harder, to prevent infections,” concludes Dr. Lundy. “More antibiotics is not always better, and using the knowledge from this study, we are optimistic that other targeted interventions may be available in the future.”
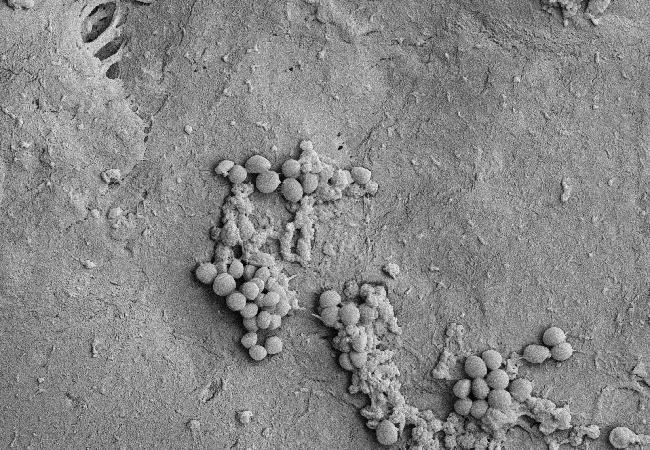
Featured image: Staphylococcus epidermidis isolate from an IPP device grown on polytetrafluoroethylene (PTFE), a common material used in devices, from in vitro biofilm bioreactor.
Advertisement
Advertisement

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Fixed-dose single-pill combinations and future therapies

Reproductive urologists publish a contemporary review to guide practice

Two recent cases show favorable pain and cosmesis outcomes

Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood

Proteinuria reduction remains the most important treatment target.

IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy

Oncologic and functional outcomes are promising, but selection is key