DEFUSE 3 results support lengthening treatment window in imaging-selected patients
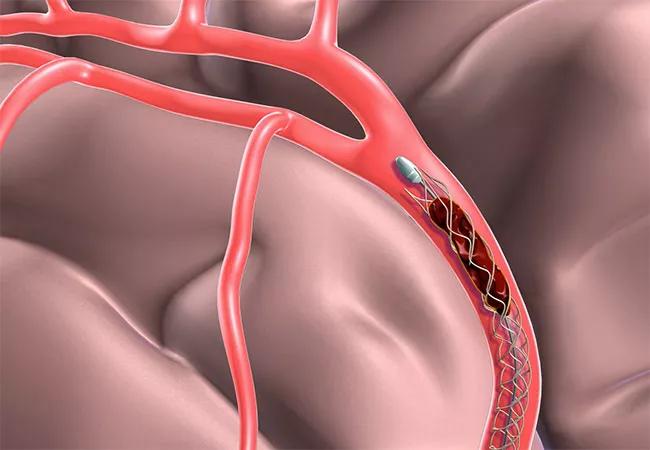
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e8206fe1-1037-4e08-b1fd-c6cc86a8a8e4/17-NEU-4426-Uchino-Defuse3-Trial-650x450_jpg)
17-NEU-4426-Uchino-Defuse3-Trial-650×450
The DEFUSE 3 trial — undertaken to determine if mechanical clot retrieval is beneficial when performed between six and 16 hours after symptom onset in patients with acute ischemic stroke selected based on imaging — was terminated early last year because of high likelihood of superiority in the intervention arm. Now the multicenter trial’s eagerly awaited results, which showed a threefold higher likelihood of recovery to functional independence with mechanical thrombectomy relative to standard medical therapy, have been presented at the 2018 International Stroke Conference and published online by the New England Journal of Medicine.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Two trials have now demonstrated that thrombectomy can confer benefit in stroke even if conducted more than six hours after symptoms start,” says Ken Uchino, MD, Director of Research and Education in Cleveland Clinic’s Cerebrovascular Center, who served as lead investigator for DEFUSE 3 at Cleveland Clinic. “We would expect criteria for performing this intervention to broaden in the coming years, further expanding treatment options for this devastating event.”
Although intravenous thrombolytic therapy (IV tPA) has been available to treat acute ischemic stroke for more than two decades, its use is limited by a restrictive time window, multiple contraindications and low rates of artery recanalization. In 2015, four randomized trials demonstrated the safety and efficacy of endovascular stroke therapy using mechanical stent retrievers within six hours of stroke onset, establishing thrombectomy as standard of care for selected patients within that time window. However, whether the treatment is beneficial when administered beyond six hours remained unanswered.
The DAWN and DEFUSE 3 trials sought to answer that question using CT or MRI selection of patients for trial qualification. The two studies were similar in many respects but differed in the following aspects:
Advertisement
Overall, DAWN was more restrictive than DEFUSE 3, with about 40 percent of DEFUSE 3 participants not meeting the DAWN selection criteria.
The results of the DAWN trial, published in November 2017 in the New England Journal of Medicine, reported superior outcomes for patients treated with thrombectomy plus standard care versus standard care alone. That trial was also terminated early for superiority in the thrombectomy group.
The prospective phase III DEFUSE 3 trial randomized patients with acute ischemic stroke within six to 16 hours of symptom onset to either endovascular thrombectomy plus standard medical therapy or standard medical therapy alone. Entry criteria included evidence of an occlusion of the intracranial internal carotid artery or proximal middle cerebral artery by CT or MR angiography as well as a target mismatch profile on CT perfusion or MR diffusion and perfusion-weighted imaging, as determined with an automated image analysis platform.
Thrombectomy could be performed using any of four FDA-approved stent-retriever devices according to physician discretion. The primary end point, modified Rankin scale score, was assessed at three months.
Begun in April 2016, the study was designed to recruit 476 patients over four years. Actual enrollment was 182 patients at the time of termination in August 2017.
At three-month follow-up, a modified Rankin scale score of 0-2, indicating ability to live independently, was achieved by 45 percent of patients in the thrombectomy group compared with only 17 percent of patients in the medical therapy group, which represents a near tripling of functional independence rates with thrombectomy. The adjusted common odds ratio for a better outcome was 3.4. The number needed to treat to prevent disability was 2.
Advertisement
Additionally, the likelihood of severe disability or death (modified Rankin scale score of 5-6) was significantly reduced in the thrombectomy group (2.2 percent) versus the medical therapy group (4.2 percent).
Rates of symptomatic intracranial hemorrhage were statistically comparable between the groups (6.5 percent with thrombectomy versus 4.4 percent with medical therapy alone).
The recanalization rate was comparable to rates in recent clinical trials of acute endovascular therapy, with 76 percent grade 2b or 3 recanalization on the Thrombolysis in Cerebral Infarction (TICI) scale.
The median NIH Stroke Scale score was 16 in each group. Median core volume was 10 mL. Median time to randomization was 11 hours after the patient was last seen normal.
There was no difference in benefit from endovascular therapy according to whether patients were randomized early (within 11 hours) or later, or whether they represented cases of “wake-up” stroke or stroke with witnessed onset.
According to Dr. Uchino, the standard of care will likely change as a result of the encouraging findings of the DAWN and DEFUSE 3 trials for mechanical intervention in selected patients up to 24 hours after symptom onset. He says more trials will likely be undertaken soon to assess further broadening the use of thrombectomy for ischemic stroke.
He notes that a different problem not so easily addressed in clinical trials is how to implement the newest findings in the existing healthcare system. The advanced imaging techniques used to qualify patients for endovascular treatment in these trials, as well as the treatment itself, are generally available only at large hospitals. Although the trials extended the window for endovascular intervention, prompt treatment is still critical for optimal outcomes.
Advertisement
“How do we rapidly match patients who are likely to benefit from advanced care with facilities that can provide it?” asks Dr. Uchino. “The next challenges include determining how to implement a system to provide patients with optimal therapy as quickly as possible.”
Advertisement
Advertisement
Guidance from the largest cohort of SEEG-confirmed insular epilepsy patients reported to date
Ethical guidance provides guardrails so medical advances benefit patients
OCEANIC-STROKE results represent long-sought advance in secondary stroke prevention
Two studies from Cleveland Clinic may help advance the technology toward broader clinical use
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
An argument for clarifying the nomenclature
An expert talks through the benefits, limits and unresolved questions of an evolving technology
Recommendations on identifying and managing neurodevelopmental and related challenges