Insights from Cleveland Clinic specialists pioneering its placement
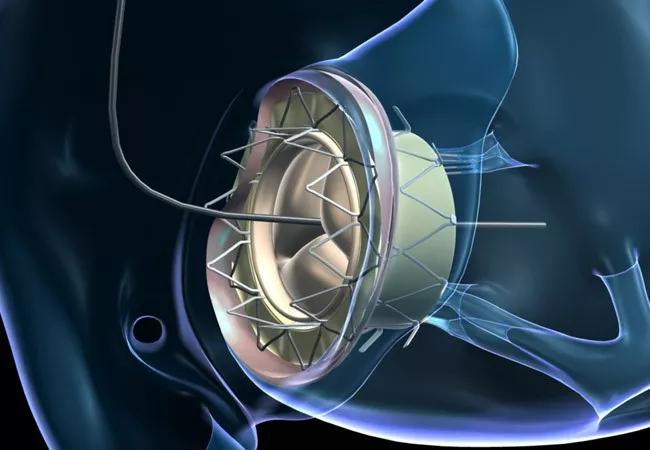
Until November 2016, there were no reports of transcatheter valve implantation at the native tricuspid annulus level in humans. That’s when Cleveland Clinic specialists performed the landmark first percutaneous implantation of a novel tricuspid valved stent directly to the tricuspid annulus in a 64-year-old woman with severe tricuspid regurgitation. They have since performed transcatheter placement of the self-expanding valved stent in a total of four patients through May 2018, published satisfactory results from long-term preclinical models (JACC Basic Transl Sci. 2018;3:67-79) and published successful results from the first two cases of human implantation of the stent (Circ Cardiovasc Interv. 2017;10:e005840).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We are satisfied with the results of this procedure in all the cases we have done to date,” says the principal author of the above papers, Jose Navia, MD, Vice Chair for Innovation in Cleveland Clinic’s Department of Thoracic and Cardiovascular Surgery. “There is every indication that this technique can be successfully used to replace faulty tricuspid valves in patients at high surgical risk.”
The novel prosthesis — known as the GATE™ tricuspid valved stent, from NaviGate Cardiac Structures Inc — addresses the need for a less-invasive treatment for tricuspid regurgitation (TR), a condition suffered by an estimated 7 million people in the U.S. and Europe. Without treatment, TR inevitably leads to right-sided heart failure and death.
Historically, TR has been ignored, and not without reason. TR is usually secondary to mitral valve dysfunction. It often presents with pulmonary hypertension, atrial fibrillation and other serious comorbidities. Mortality for surgical repair or replacement of the tricuspid valve can be as high as 35 percent.
At one time, it was believed that TR secondary to mitral valve dysfunction would correct itself after mitral valve replacement or repair. This has been proven false.
TR is a self-exacerbating condition, with mild or residual TR eventually progressing to the torrential and fatal stage.
“Functional tricuspid regurgitation enlarges the annulus, causing a reverse flow of venous blood from the right heart — blood that should be going to the lungs,” says Samir Kapadia, MD, Section Head of Interventional Cardiology at Cleveland Clinic and a co-author of the papers cited above. “It’s not possible to completely eliminate torrential tricuspid regurgitation with current treatments.”
Advertisement
It is in this context that the GATE stent system was developed, with the incorporation of Cleveland Clinic intellectual property. (Dr. Navia is the inventor of patents related to the device, and both he and Dr. Kapadia are on NaviGate Cardiac Structures’ scientific advisory board and own stock in the company.)
The GATE device is a biological valved stent with a lining designed to support the pericardial membrane wall to protect its integrity and prevent paravalvular leakage. The stent comes in five sizes (36, 40, 44, 48 and 52 mm) to meet various anatomical contingencies. It is shaped somewhat like a truncated cone, to address the fact that in most patients with TR, the ventricular annulus is more dilated than the atrial side.
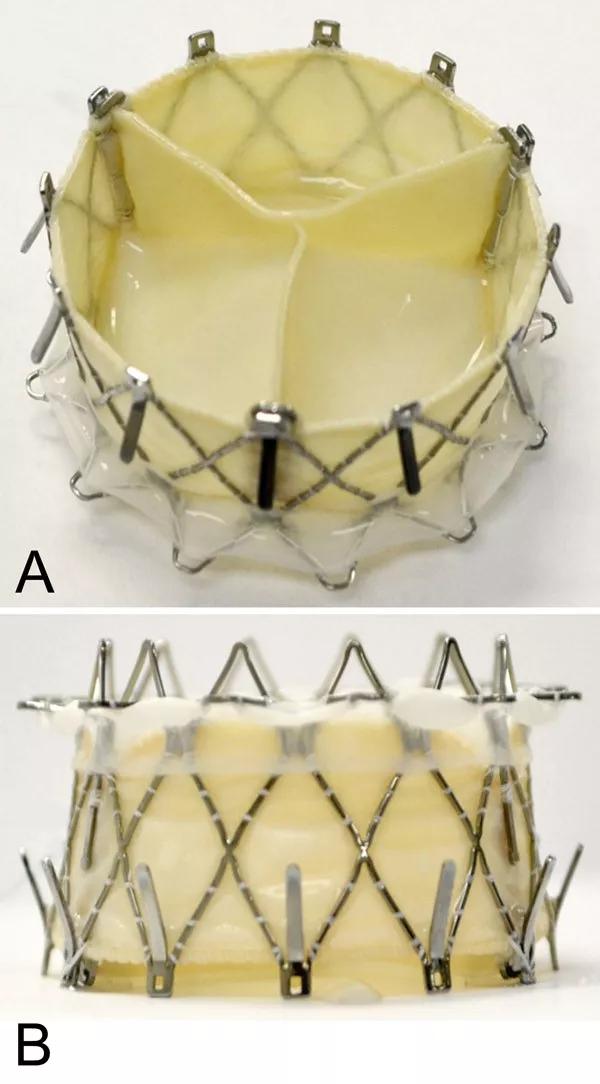
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/965b0613-9cef-4200-a494-a3daae4ec90f/18-HRT-4694-GATE-tricuspid-valved-stent-Inset_jpg)
Photos of the GATE valved stent showing the outflow (left ventricle) view and side view. Reprinted from Navia et al., JACC Basic Transl Sci. 2018;3:67-79.
When assembled, the bioprosthesis undergoes a proprietary dehydration process in which all glutaraldehyde and most of its water are removed, after which the dehydrated valve is sterilized by ethylene oxide exposure. This process, which originated from Cleveland Clinic research, is intended to extend the longevity of the valvular mechanism during storage.
The bioprosthesis is crimped and packed into the end of an L-shaped delivery device. It is transluminally delivered to the tricuspid annulus under the guidance of fluoroscopy and intracardiac echocardiography in a hybrid operating room. Delivery can be via transatrial access (through a mini-thoracotomy) or transjugular access.
Advertisement
Positioned coaxially in the annulus, the valve self-expands and is held in place by barbed graspers at one end and radial pressure at the other. The valve has been designed to minimize interference with adjacent chambers, but precise placement along the plane of the annulus is critical.
Expansion and placement of the bioprosthesis takes only seconds and is performed on the beating heart without rapid ventricular pacing.
All four cases performed by the Cleveland Clinic team have been done under a compassionate-use protocol.
The first patient — the 64-year-old woman mentioned at the start of this story — had a severely dilated tricuspid valve, multiple hospitalizations for refractory right heart failure, severe pulmonary hypertension and many additional comorbidities, along with prior chest radiation for breast cancer. She underwent implantation of the valved stent via transatrial access. At five-month follow-up, she demonstrated functional improvement and her severe TR had been reduced to mild to moderate.
The second patient was a 78-year-old man with a history of three coronary artery bypass surgeries, a mitral valve repair and two tricuspid valve repairs. He also had diabetes mellitus, atrial fibrillation, obstructive lung disease and chronic kidney disease. His prosthesis was delivered via the transjugular vein and anchored in the tricuspid annuloplasty ring from one of his prior surgeries. He was faring well at one-year follow-up, with excellent valve function.
A third patient demonstrated that the bioprosthesis can be used in patients with pacemaker leads traversing the tricuspid valve. This 79-year-old woman received her implant through a transatrial approach and was discharged with a well-functioning valve. She was able to return for clinical assessment from her distant home state approximately a month after intervention.
Advertisement
Transatrial delivery was again used in the fourth patient, a 77-year-old woman who has had her torrential TR reduced to a mild backflow and showed excellent valvular function at one-month follow-up.
Drs. Navia and Kapadia say the Cleveland Clinic heart team applies the following criteria to identify candidates for the GATE stent:
“The procedure still poses some technical challenges in achieving a complete seal, especially in patients with severe annular dilation,” notes Dr. Navia. “Work is underway to further optimize the delivery system at the point of distal angulation.”
Despite those challenges, he and his counterparts at the handful of other U.S. and European centers that have implanted the GATE bioprosthesis under compassionate-use protocols remain bullish on the technology. The company developing the device is now gathering data to enable the launch of an early feasibility clinical trial.
Advertisement
Advertisement

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more

CD36 loss-of-function variant accounts for large portion of risk in this population