Two weeks of therapy yields sustained benefits
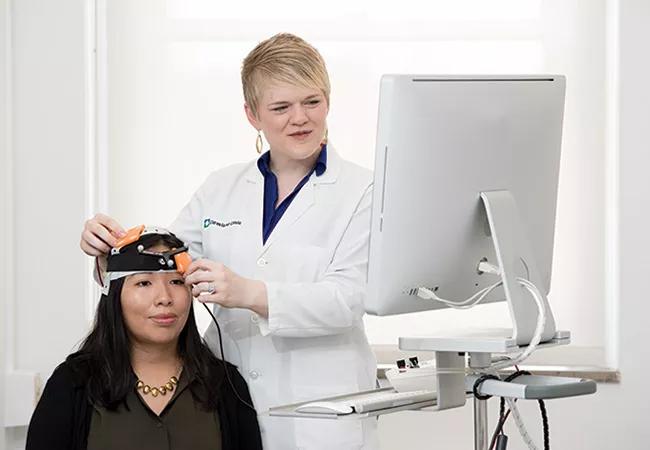
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/76350ecd-be3b-4d1b-9895-332ee004dfb6/17-NEU-4012-Potter-650x450_jpg)
17-NEU-4012-Potter-650×450
Patients with long-standing incomplete tetraplegia realized gains in motor function below the level of injury after a two-week program pairing transcranial direct current stimulation (tDCS) with massed practice training in a new pilot study. The gains were still evident three months following the intervention, and motor map characteristics also showed increased excitability of residual pathways.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Results of the randomized study, conducted at Cleveland Clinic, were reported as a platform presentation at the Academy of Spinal Cord Injury Professionals (ASCIP) educational conference in September 2017. The study abstract was selected from more than 200 entries to be presented as the conference’s prestigious Jayanthi Lecture.
“In this preliminary study, we observed significant functional benefits in these very disabled patients in the chronic stage of injury after only a short intervention,” says Kelsey Potter-Baker, PhD, a research scientist in Cleveland Clinic’s Department of Biomedical Engineering and lead author of the study. “One patient, who for years had been unable to use a computer keyboard, could do so following treatment. This was life-changing for her.”
The full-length study report has been published in the Journal of Spinal Cord Medicine.
tDCS has emerged as a promising intervention for rehabilitation after neural injury. Incomplete spinal cord injury usually involves neural reorganization favoring muscles above the level of injury to the detriment of those below, which become weak and poorly amenable to physical therapy. tDCS is thought to be able to enhance excitability of neurons and facilitate brain plasticity that can contribute to recovery of the weak muscles.
tDCS has been applied to patients following a stroke with favorable results, but the Cleveland Clinic study is its first reported use for a substantial period in patients with spinal cord injury.
Advertisement
The double-blind, randomized study involved 12 subjects with chronic incomplete spinal cord injury who underwent two hours of physical therapy (massed practice training) five days a week for two weeks coupled with either tDCS or sham stimulation. (Data on just eight subjects were available at the time the Journal of Spinal Cord Medicine paper was submitted.)
All subjects had tetraplegia with injury to the cervical region and were more than 20 months past the time of injury, which is considered beyond the period when spontaneous recovery can be expected.
tDCS involved placing the anodal electrode over the primary motor cortex at the determined site of the weaker muscle lying below the level of injury. Stimulation (2 mA) was applied during the first 30 minutes of the first hour of massed practice training and again for the first 30 minutes of the second hour.
Neurophysiologic and functional measures were assessed before, after and three months following the intervention.
The study found that subjects who received training paired with tDCS had a greater increase in strength of weak proximal muscles (15 vs. 10 percent), wrist muscles (22 vs. 10 percent) and hand muscles (39 vs. 16 percent) compared with the sham stimulation group. Benefits in both groups were retained at three-month follow-up, with a slight advantage seen in the intervention group.
Neurophysiologic changes observed in the intervention group but not in the sham group included decreases in strong muscle map volume and a more focused weak muscle motor map that relocated toward its expected location — changes believed to reflect the observed functional improvements.
Advertisement
“Our findings provide evidence that stimulation helps restore cortical representations of paralyzed and weak muscles, and strongly suggest the potential for adaptive plasticity long after an injury,” says Dr. Potter-Baker.
tDCS, a commercially available device, is battery-operated and applied using a headband, allowing the patient to move freely during stimulation.
The intervention is noninvasive and inexpensive and can easily be added to a patient’s normal clinical care, notes Dr. Potter-Baker, who is shown demonstrating use of the tDCS device in the photo above. Each study subject’s intervention involved physical therapy designed by physical therapists to target individual patient goals, which is typical of routine rehabilitation, she adds.
As a result of this promising study, Dr. Potter-Baker is now working toward conducting a larger phase 2 clinical trial with the aim of obtaining FDA approval of tDCS for this indication. Her team is also investigating how to optimize benefits in terms of the length of intervention and the tDCS dosage.
She speculates that the therapy may need to be repeated periodically to maintain and possibly build on gains.
Advertisement
Advertisement
Guidance from the largest cohort of SEEG-confirmed insular epilepsy patients reported to date
Ethical guidance provides guardrails so medical advances benefit patients
OCEANIC-STROKE results represent long-sought advance in secondary stroke prevention
Two studies from Cleveland Clinic may help advance the technology toward broader clinical use
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
An argument for clarifying the nomenclature
An expert talks through the benefits, limits and unresolved questions of an evolving technology
Recommendations on identifying and managing neurodevelopmental and related challenges