HNS device offers new solution for those struggling with CPAP
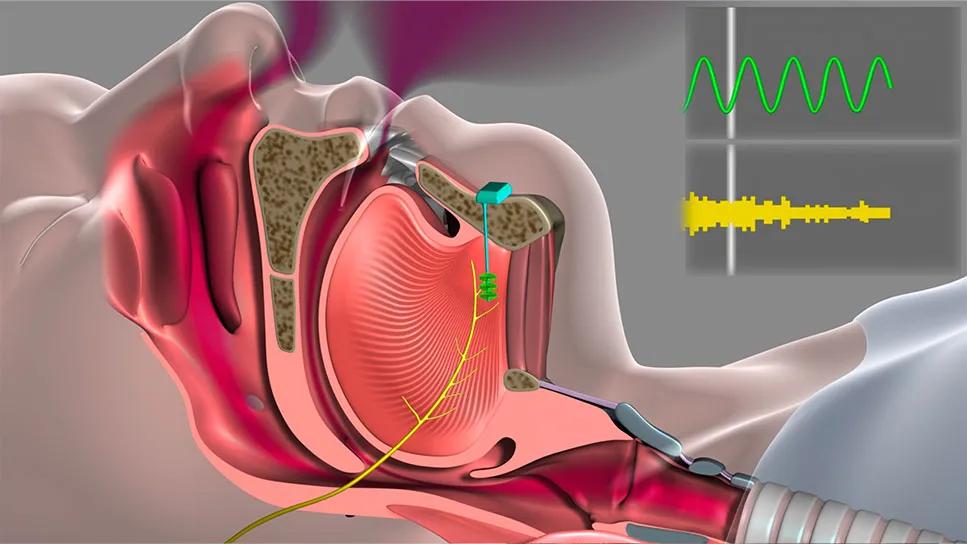
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4e64adcf-4c90-4f01-8a14-5c2b3d80cc1b/25-HNI-5887712-HypoglossalNerveStim-CC-animationStill-CQD_967x544)
Diagram of Inspire Device
In March 2023, the U.S. Food and Drug Administration approved the Inspire® device, a hypoglossal nerve stimulator (HNS), for use in pediatric patients with Trisomy 21 who experience obstructive sleep apnea (OSA) and cannot tolerate continuous positive airway pressure (CPAP) therapy.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Patients with Trisomy 21 typically have larger body mass, a smaller jaw and a flatter midface. “These features help create a perfect storm for obstructive sleep apnea,” says Brandon Hopkins, MD, Section Head of Pediatric Otolaryngology-Head and Neck Surgery and Surgical Director of the Pediatric Center for Airway, Voice and Swallowing at Cleveland Clinic Children's. “Over the years, removing the tonsils and adenoids has been highly successful in a typically developing child, but with Trisomy 21, the chances of sleep issues are really high.”
Dr. Hopkins notes that poor sleep and difficulty concentrating worsen the developmental challenges these patients already face. In the past, removing their tonsils and adenoids helped, but it did not completely solve the problem, and CPAP can be a struggle in this population.
The following year after the FDA approval, Cleveland Clinic performed its first implantation of the Inspire® device in a pediatric patient with Trisomy 21 in March 2024. Cleveland Clinic is still currently one of just a few hospitals to offer the procedure in a seven-state region.
The patient was diagnosed with OSA at 9 years old and had tried using the CPAP device three different times. However, she found the mask uncomfortable and would often remove it in the middle of the night. By age 11, she had stopped using the device despite being jolted awake every time she stopped breathing.
“Before surgery, the patient had pauses or slowing in her breath 16 times an hour,” says Dr. Hopkins. “Just imagine someone shaking your knee 16 times an hour when you’re sleeping. CPAP blows up the pharynx like a balloon to open the airway. The Inspire® device works the opposite way by moving the tongue forward to allow the person to breathe normally.”
Advertisement
In addition to Dr. Hopkins, the multidisciplinary surgical team also included Swathi Appachi, MD, a staff member with Cleveland Clinic Children’s Hospital and the Head & Neck Institute.
The device was implanted during an outpatient procedure, and the patient was put under general anesthesia. An incision was made in her upper neck near her jaw to locate the part of the nerve that moves the tongue and place an electric stimulation cuff around it. Another incision was made in her upper chest, where the device and a sensor were placed between her ribs, and then everything was connected.
“One of the unique aspects of how we do these implantations at Cleveland Clinic is that our Sleep Medicine Team actually comes into the operating theater to program the device and make sure the device is functioning properly,” says Dr. Hopkins. “That’s not typically done for our adult patients, but for patients with developmental disabilities or who aren’t able to communicate as well as the general population, it’s a great option. Plus, these patients will work closely with our Sleep Medicine Team postoperatively to fine-tune and figure out the settings that work best for the patient. So having the sleep specialists involved from the beginning really provides them with great perspective and understanding of each individual patient.”
After the surgery, the patient was released later that same day. After a month of healing, Danielle saw Vaishal Shah, MD, MBBS, MPH, Interim Director of the Sleep Disorders Center, who activated, programmed and adjusted the device over several weeks. She then uses a remote control to turn on the device before going to sleep.
Advertisement
The device has helped decrease her breathing pauses down to twice per hour or lower.
“We've been pleasantly surprised with just the drastic improvement that these kids are getting with this device as compared to their baseline,” says Dr. Hopkins. “Even after they were put on CPAP, their breathing pauses have been less on Inspire®, and they tolerate it much better than CPAP.”
Since the initial surgery in March 2024, Cleveland Clinic has performed several more Inspire® implantations for pediatric patients with Trisomy 21.
“All of these patients have responded really well,” says Dr. Hopkins. “All but two of these kids went home the same day, one of the patient’s developmental disabilities were a little more severe and the family wanted to stay overnight, and the other patient was 11 years old, which I believe is the youngest patient to receive the device in the United States and we wanted her to stay a little longer for observation.”
Very careful consideration by the team was given to the initial patient when selecting her for the procedure.
“We thought she was ideal candidate for Inspire®,” says Dr. Hopkins. “These kids often have higher body fat percentages, but this patient was not considered obese, she is high functioning, so she’s able to communicate well and let us know if something is working or not and her sleep study was severe enough that we thought this procedure could help with her daytime sleepiness and would have clinically relevant results.”
While the initial patient was considered high functioning, Dr. Hopkins says that cognitive ability should not preclude patients with Trisomy 21 from receiving the device. “In those cases, programming the device might need to be done a little differently,” he explains. “At Cleveland Clinic, we’ve used a drug-induced sleep endoscopy, where instead of programming somebody in the office and getting feedback verbally, we’ll have them go back to sleep and insert an endoscopic camera through their nose to see how the device is working in real-time.”
Advertisement
Although these pediatric patients are still growing, Dr. Hopkins doesn’t anticipate they will need a revision surgery to readjust the sensor placement. He explains that, while his primary concern initially was the distance between the neck incision and the chest incision, the sensor leads provide considerable redundancy. He also believes that any growth between the neck and chest will not be significant enough to have an impact.
“Most of these 13- and 14-year-old kids with Trisomy 21 are already near full growth at that point already, so we don’t expect the amount of growth – something like six inches – that would be enough to make an impact,” says Dr. Hopkins. “However, like all of the other patients who receive the device, we will have to go back in at some point down the road to replace the battery. They typically last around 10 years, but the next generations of the device will likely have longer battery lives, and the new 5th generation device only uses a stimulation lead instead of the two leads the 4th generation has.”
Dr. Hopkins expects the FDA to eventually approve the device for pediatric patients beyond just the ones with Trisomy 21. He says that providers should start thinking about if they have children with OSA or who have had a tonsillectomy/adenoidectomy and are still symptomatic. “I think this is eventually going to be on the table for more populations than just the patients with Trisomy 21,” he says.
Advertisement
Advertisement
Looking at short-term outcomes in a high-risk population
Analysis of HNSCC patients shows HPV status to be predictive of higher abundance of oncobacteria within the tumor
Case study illustrates the potential of a dual-subspecialist approach
Evidence-based recommendations for balancing cancer control with quality of life
Study shows no negative impact for individuals with better contralateral ear performance
Patient with cerebral palsy undergoes life-saving tumor resection
Specialists are increasingly relying on otolaryngologists for evaluation and treatment of the complex condition
Detailed surgical process uncovers extensive middle ear damage causing severe pain and pressure.