Surprise findings argue for caution about testosterone use in men at risk for fracture
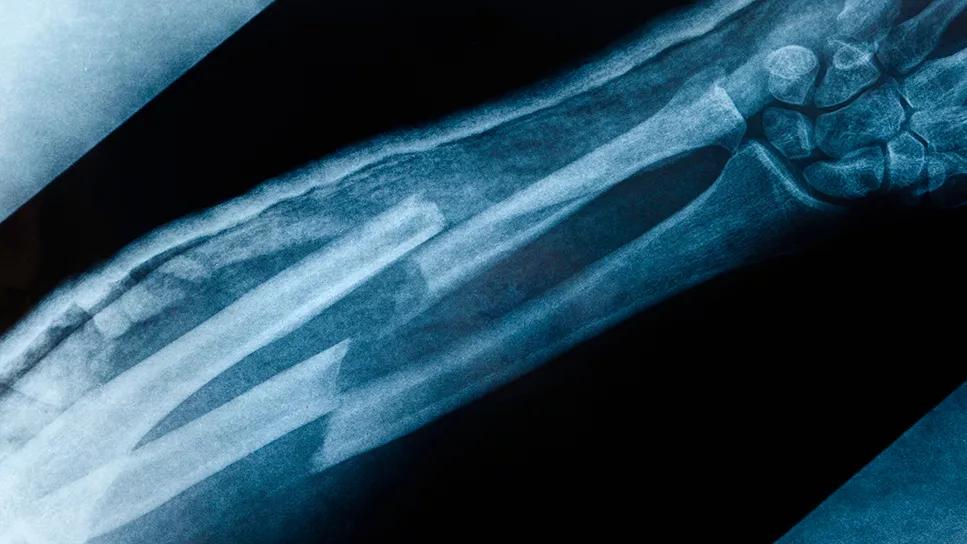
The large TRAVERSE study — which found testosterone replacement therapy was not associated with an increase in major adverse cardiovascular events in men aged 45 to 80 with hypogonadism — has yielded a surprising secondary finding: testosterone treatment was associated with an increased risk of fractures compared with placebo.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The result, recently published in the New England Journal of Medicine (2024;390:203-211), defied expectations of a reduction in fracture risk based on past evidence of improvement in bone structure and bone quality following testosterone therapy in hypogonadal men.
“Despite the unexpected nature of these findings, their consistency was strong,” says senior author and TRAVERSE study chair Steven Nissen, MD, Chief Academic Office of Cleveland Clinic’s Heart, Vascular & Thoracic Institute. “This suggests there’s something going on here that is biologically important. If men have a history of osteoporosis, they probably shouldn’t be taking testosterone, and they certainly shouldn’t take testosterone to reduce their risk of fracture because it appears to actually increase that risk.”
“This study addresses an important knowledge gap — whether testosterone replacement in men with hypogonadism reduces fracture risk,” adds Dennis Bruemmer, MD, PhD, Director of the Center for Cardiometabolic Health in Cleveland Clinic’s Section of Preventive Cardiology. “Although there is good evidence that testosterone increases bone mineral density, testosterone replacement did not reduce fracture incidence in this study, which suggests it may even increase fracture risk. These results confirm our incomplete understanding of testosterone’s role in bone health and warrant further study of underlying mechanisms and how testosterone directly affects bone remodeling.”
The analysis was a prespecified substudy of TRAVERSE, a double-blind, randomized, placebo-controlled trial primarily assessing cardiovascular outcomes with testosterone treatment. The primary study report, published last year in the New England Journal of Medicine, provided reassurance about the cardiovascular safety of testosterone therapy over the typical duration of treatment in men in whom it is indicated (see study recap here).
Advertisement
This substudy examined fracture incidence among 5,204 men aged 45 to 80 years with hypogonadism and preexisting cardiovascular disease or cardiovascular risk factors who were randomized to receive testosterone gel or placebo gel daily for a median follow-up of 3.19 years.
After adjudication of all reported fractures, the researchers found that 3.50% of men treated with testosterone experienced a clinical fracture compared with 2.46% of those on placebo (hazard ratio = 1.43; 95% CI, 1.04-1.97). By year 3, the cumulative incidence of clinical fracture was 3.8% in the testosterone group versus 2.8% in the placebo group.
Testosterone treatment was associated with a numerically higher incidence of fractures across all prespecified fracture endpoints.
The most common fracture sites were the ribs, wrist and ankle. Over 80% of the fractures occurred with trauma, most often due to falls. The fracture types and anatomical locations were typical of osteoporotic fractures seen in older men.
Because the finding of greater fracture risk was unexpected, the study was not designed to assess mechanisms by which testosterone therapy might increase fracture incidence. The authors of the substudy, led by endocrinologist Peter J. Snyder, MD, note that potential mechanisms are unclear but may relate to detrimental effects of testosterone on cortical bone.
The study had several strengths, including its large sample size, placebo control, prospective design, median three-year follow-up and central adjudication of fracture outcomes. However, limitations included suboptimal adherence, smaller-than-expected increases in testosterone levels with treatment, and lack of assessments for falls, bone density and structural integrity.
Advertisement
An editorial accompanying the study speculated that the increase in fractures among testosterone recipients might be due to greater physical activity related to testosterone’s effects. Dr. Nissen finds this implausible. “The data do not support that,” he says. “There was an excess of nonimpact fractures that was just about the same as the increase in all clinical fractures. Moreover, the efficacy of testosterone in these men for everything else we measured was very moderate. So it’s simply unlikely that they went out and suddenly started engaging in behavior with much higher fracture risk.”
While the editorial calls for more research to clarify the skeletal effects of testosterone therapy in men with hypogonadism, Dr. Nissen says it’s unlikely another similarly rigorous investigation of this question will be conducted, given logistical and cost challenges.
“These findings may be the best answer we will get,” he concludes. “So I would urge men to be careful about the use of testosterone in settings where they are at risk for fracture based on having osteoporosis or partaking in activities with a high fracture risk. Caution is warranted in light of these findings.”
Advertisement
Advertisement

Unlike earlier pills, new drugs do not cause liver toxicity
But findings apply only to middle-aged or older men with confirmed hypogonadism

Reproductive urologists publish a contemporary review to guide practice

American Urological Association presentation outlines the latest evolution of treatment protocols

How a multicenter consortium aims to change the research landscape

Early, individualized diagnosis and comprehensive management key to preserving fertility

Testosterone deficiency can prove challenging to diagnose and treat

Male factors play a role in about half of all infertility cases, yet men often are not evaluated