10-year outcomes favor autologous over implant
Radiation therapy and breast reconstruction after mastectomy don’t always go well together. Rates of infection, dehiscence, skin and/or flap necrosis, and hematoma are higher in patients that undergo reconstruction and have post-mastectomy radiation therapy. In those patients that have reconstruction with a tissue expander/implant (TE/I), radiation is associated with higher rates of implant extrusion, leak or capsular contracture, while those that have autologous reconstruction (AR) are at risk for hernias as well.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Toxicity profiles vary by technique and timing, and data currently available in the literature provide little guidance on determining the optimal treatment approach, says Chirag Shah, MD, Director of Clinical Research and Breast Radiation Oncology in Cleveland Clinic’s Department of Radiation Oncology.
In response, Dr. Shah and colleagues at Cleveland Clinic recently compared rates of complications in patients who had post-mastectomy radiation therapy both before and after AR or TE/I.
“We suspected that radiation to a TE/I, regardless of timing, would portend worse outcomes,” says Dr. Shah. “Our study confirmed this. For patients requiring radiation after mastectomy, AR was associated with fewer trips back to the operating room for complications and fewer reconstruction failures.”
Results of this study will be shared in an oral presentation at the 2018 ASTRO Annual Meeting in San Antonio.
The study included 230 patients that had 233 breast reconstructions. The patients had either AR or TE/I, either before or after radiation therapy, between 2000 and 2008. Median follow-up was 7.6 years. Age, BMI, and rates of active smoking, diabetes and hypertension were similar among the groups. Reconstruction was performed at the time of mastectomy, before radiation therapy, in 81 percent of patients.
Overall, rates of reconstruction failure (resulting in converting to another reconstruction technique or a flat chest wall) and complications requiring re-operation were significantly higher with TE/I as compared to AR (Table).
Advertisement
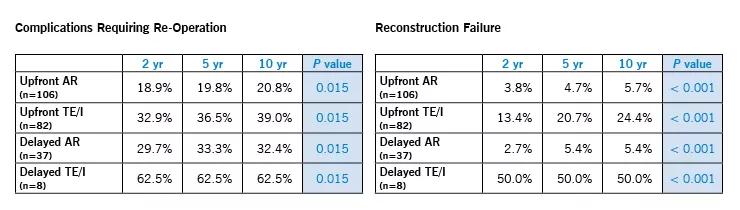
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9cc9368e-033d-4b87-9f83-562ca31c1486/18-CNR-4330-charts_740x210_jpg)
On multivariate analysis, the most significant predictors of complications requiring re-operation were:
The only significant predictor of reconstruction failure was TE/I (Odds Ratio 5.4; P < 0.001).
However, the rate of failure was not significantly different between the four groups when wound infection was excluded (P = 0.156).
Most complications requiring re-operation occurred within the first two years following treatment, but reconstruction failure occurred up to 10 years after reconstruction.
“Many patients undergoing mastectomy get expanders and/or implants whether or not they have radiation therapy,” says Dr. Shah. “We may need to rethink that paradigm.”
These findings indicate that radiation therapy in patients with AR results in fewer complications and reconstruction failures than radiation therapy in patients with TE/I. Yet risk of failure with TE/I can be reduced by minimizing risk of infection.
Radiation oncologists should discuss treatment options in a multidisciplinary, collaborative group with breast surgeons and plastic surgeons, ensuring plans are in place for managing reconstruction risks, says Dr. Shah.
Advertisement
Advertisement

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units

Addressing rare disease and challenging treatment course in an active young patient