How far we’ve come with implant technology
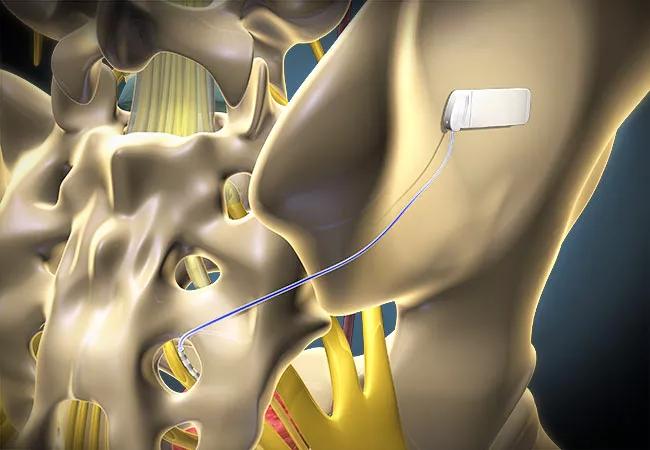
Nearly 30 years later, one of the first patients ever to be implanted with a sacral neuromodulation (SNM) device for voiding dysfunction continues to be symptom-free. Age 52 when she received the implant in 1991 as part of a clinical trial, the now 81-year-old has been able to urinate normally with no additional treatment aside from battery changes every few years.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The trial was for patients who had idiopathic urinary retention or severe urge incontinence,” says Howard B. Goldman, MD, Vice Chair at Cleveland Clinic’s Glickman Urological & Kidney Institute. “This patient had idiopathic urinary retention. The only thing she could do was catheterize herself multiple times a day. In addition to increasing her risk of infection, catheterization was not what she wanted to do for the rest of her life.”
Steven W. Siegel, MD, then Head of the Section of Female Urology and Urodynamics at Cleveland Clinic, implanted the SNM device, an early version of what would become Medtronic’s InterStim™ technology. InterStim was approved by the Food and Drug Administration in 1997. The technology works by stimulating a sacral nerve, thereby “hacking” into the neural system that controls bladder-to-brain communication and normalizing bladder activity.
The patient was one of 28 to be implanted at Cleveland Clinic during the trial.
“Within days of getting the implant, the patient was able to void on her own,” says Dr. Goldman. “That continued for a number of years until the battery in the generator started to wear out.”
Changing the battery requires changing the entire stimulus generator, the silver-dollar-sized pack implanted just within the patient’s buttock. The lead — the wire that goes under the skin, through the tailbone and alongside the third sacral nerve — stays in place. Patients are sedated for this outpatient procedure, which takes about 15 minutes.
The patient in this case has undergone the battery-changing procedure four times, in 2000, 2007, 2014 and 2020. In 2014 Dr. Goldman also changed the lead, which had begun to show wear after 23 years.
Advertisement
“We expect to get six to seven years of battery life each time,” says Dr. Goldman. “The patient knows when it’s time to change the battery because her voiding problems start to recur. When the generator is at full power, her urination is totally normal.”
SNM is now the standard of care for patients with idiopathic urinary retention and refractory overactive bladder. More than 325,000 patients have been implanted worldwide.
“With the original devices, we needed to cut all the way into the fascia and suture the lead in place,” says Dr. Goldman. “About 15 years ago, we started using a new type of lead that had little tines on it. Now suturing isn’t necessary. We can do the insertion almost percutaneously.”
Over the years, generator packs have become thinner and smaller. Their power now can be programmed remotely with a handheld device.
“I still have an SNM programmer from 1997,” says Dr. Goldman. “It’s the size of a suitcase, with a huge thing you put over the patient’s body and a spool of paper for printing out settings. Now the programmer looks and operates like a smartphone.”
In addition, SNM devices today come in models that are rechargeable and MRI-safe, made by both Medtronic and a newer company, Axonics.
Historically, to comply with FDA regulations, patients had SNM devices surgically removed before having MRIs. Now patients can avoid this disruption in their urology care as well as the exorbitant cost. MRI-safe SNM devices may be a game-changer for patients with neurological conditions, notes Dr. Goldman, particularly those with multiple sclerosis, who may experience overactive bladder and need more frequent MRI scans.
Advertisement
“Cleveland Clinic was and continues to be a trailblazer in SNM,” says Dr. Goldman, adding that Cleveland Clinic was first in the U.S. to implant the new Medtronic MRI-safe SNM device as well as a rechargeable SNM device. “We continue to implant about 150 patients a year — thousands of patients since the 1990s.”
Researchers at Cleveland Clinic also have been leaders in understanding just how SNM works. They have used functional MRI to measure changes in brain activity in patients with overactive bladder. And they continue to study alternate types of neuromodulation, such as using a tibial nerve implant, and new patterns of stimulation for better outcomes.
“SNM will continue to improve, but it’s already effective in reducing bladder symptoms for the vast majority of patients, without side effects,” says Dr. Goldman. “Our 81-year-old patient — one of the longest-followed patients with SNM for voiding dysfunction — is a prime example. Instead of catheterizing, she has spent 30 years with a normal-functioning lower urinary tract. Her story shows the length and breadth of change that one intervention can produce in a patient’s quality of life.”
Advertisement
Advertisement

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Fixed-dose single-pill combinations and future therapies

Reproductive urologists publish a contemporary review to guide practice

Two recent cases show favorable pain and cosmesis outcomes

Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood

Proteinuria reduction remains the most important treatment target.

IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy

Oncologic and functional outcomes are promising, but selection is key