Why retina specialists should get comfortable with this imaging tool
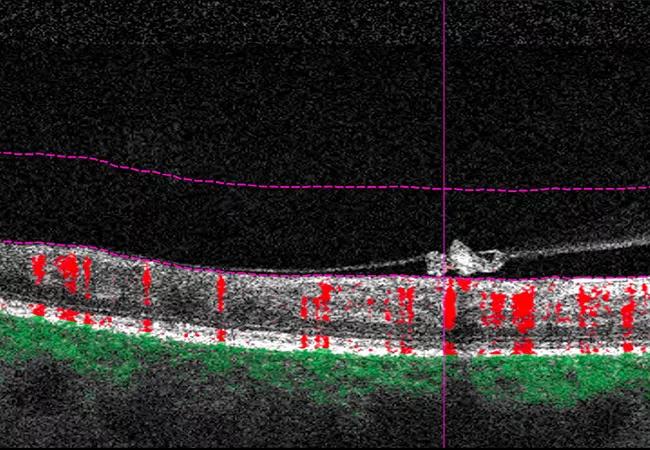
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3f8052f9-9377-4419-bda4-f659b286edd2/23-EYE-3929961-OCTA-in-DR-and-AMD-Hero_jpg)
23-EYE-3929961-OCTA-in-DR-and-AMD-Hero
Optical coherence tomography angiography (OCT-A) can produce beautiful images of vascular networks in the retina and choroid. But in real practice, OCT-A images often appear dark and unclear.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“I don’t think that OCT-A will replace fluorescein angiography (FA) anytime soon, especially in my uveitis practice, but I think it can be a useful adjunct in clinical practice,” says Danny Mammo, MD, a vitreoretinal disease and uveitis specialist at Cleveland Clinic Cole Eye Institute.
Dr. Mammo presented on practical uses for OCT-A at the 2023 Cole Eye Institute Retina Summit in New Orleans, a prelude to the 2023 Association for Research in Vision and Ophthalmology (ARVO) annual meeting.
“OCT-A can improve detection of choroidal neovascularization (CNV), specifically type 1, where sensitivity is close to 100%,” he says.
OCT-A identifies retinal and choroidal vessels by detecting the motion of red blood cells. En face images of vascular networks can be correlated with B-scan images to pinpoint location and flow. B-scan images can be segmented to display en face images of the different layers:
Outer retina to choriocapillaris (ORCC) slabs combine views of the outer retina and choriocapillaris, which is especially helpful for identifying choroidal neovascular membranes.
While OCT-A requires high-speed instruments and longer processing times, the technology provides 3D images of the deep retina and choroid not visible by FA. In addition, OCT-A does not rely on dye injection.
Advertisement
“We’ve had two shortages of fluorescein in the past few years — one during the COVID-19 pandemic and one when a manufacturer stopped producing the dye,” says Dr. Mammo. “Also, satellite clinics don’t always have FA available, so it makes sense for retina specialists to get comfortable using OCT-A to help manage patients.”
In his clinic, Dr. Mammo uses OCT-A when evaluating and managing patients referred for age-related macular degeneration (AMD) or diabetic retinopathy. Here he shares some practical uses of the imaging technology.
OCT-A can help retina specialists determine if an exudative choroidal neovascular membrane (CNVM) is present. It can help them examine pigment epithelial detachments (PEDs) and recognize broad, low-lying PEDs.
In one example shared by Dr. Mammo, a 74-year-old patient with a history of dry AMD in the right eye and wet AMD in the left eye presented with new metamorphopsia of the right eye. OCT (compared with an OCT six weeks earlier) demonstrated a larger PED with overlying subretinal hyper-reflective material (SHRM).

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/39d5d2dd-0130-49a7-863e-515368aa594d/23-EYE-3929961-OCTA-in-DR-and-AMD-1)
OCT, right eye, of a 74-year-old with history of dry AMD in this eye and wet AMD in the fellow eye.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e8784def-064b-4bc1-8d27-37d7fde627f3/23-EYE-3929961-OCTA-in-DR-and-AMD-2)
OCT of same patient six weeks earlier.
On a segmented ORCC slab, red flow within the PED suggested that the overlying SHRM was likely an active CNVM — even though subretinal or intraretinal fluid was not evident.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/09656ee5-2e76-4868-a65f-aaa1943b7c50/23-EYE-3929961-OCTA-in-DR-and-AMD-3)
OCT-A confirms the presence of CNVM.
“You have to be careful when looking at OCT-A,” says Dr. Mammo. “Just like FA can have some questionable areas (Is it leakage or not?), there easily can be segmentation errors in OCT-A of patients with large PEDs. Large areas of geographic atrophy can mimic membranes. They can fool you into thinking that you’re seeing membranes when it’s really just underlying choroidal vessels appearing in the superficial slabs.”
Advertisement
Risk stratifying patients with dry AMD will become more relevant as more dry AMD therapies become widely available.
“Can complement-inhibitor drugs potentially cause a CNVM? Some are concerned about that risk,” says Dr. Mammo. “OCT-A may be helpful in learning if having a net [a network of neovascular vessels] increases risk for CNVM or if, as some have reported, nets actually slow down adjacent or overlying geographic atrophy.”
One study has reported that patients with wet AMD in one eye had a 6%-27% chance of having a nonexudative net in the fellow eye. Over time, the incidence of exudation in these eyes was 20%-80%.
Another study, which characterized nuanced exudation in patients receiving pegcetacoplan, reported that new-onset exudative AMD in the study eye was associated with baseline exudative AMD in the contralateral eye as expected, but also a double-layer sign, suggestive of a nonexudative macular neovascularization lesion in the study eye.
“In this study, a double-layer sign was suggestive of a nonexudative net but was not confirmed with OCT-A,” notes Dr. Mammo. “We need to look at the OCT-As of these patients to see if they can help guide us in stratifying these patients for AMD treatment.”
There are other diseases that can present with subretinal fluid and cavitary lesions of the retina with or without CNVM.
“OCT-A can be instrumental in deciding whether to treat or observe these lesions,” says Dr. Mammo.
For example, one patient referred for central serous retinopathy had OCT-A imaging that revealed something else on the ORCC slab: green flow within the PED. The imaging confirmed that instead of just subretinal fluid due to central serous retinopathy, the patient had a CNVM.
Advertisement

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7051c73f-cea4-46b0-884d-f3fbf0824ab2/23-EYE-3929961-OCTA-in-DR-and-AMD-4)
OCT-A reveals a CNVM in a patient with central serous retinopathy.
Another patient, a 44-year-old with macular telangiectasia, presented with new vision changes. The ORCC slab revealed flow in a new subretinal lesion with a net. He was treated, and his CNVM resolved.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/76f64f3e-36ca-4afe-bd6c-8333e93bdf62/23-EYE-3929961-OCTA-in-DR-and-AMD-5)
OCT rasters of the right eye of a 44-year-old with idiopathic macular telangiectasia.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/20f251dd-fa5e-4e41-bc78-596f4e5dda97/23-EYE-3929961-OCTA-in-DR-and-AMD-6)
OCT-A ORCC slab reveals flow in the subretinal lesion corresponding to the apparent CNVM visualized on the en face slab.
“One of my favorite things to do when I am referred AMD patients is to diagnose something other than AMD,” says Dr. Mammo.
OCT-A can help diagnose these other diseases.
A 71-year-old male was referred to Dr. Mammo for 20/40, 20/50 vision. Imaging was unremarkable. Only mild drusen appeared on the OCT. On OCT-A, the superficial and deep retinal slabs appeared normal. However, the choriocapillaris slab showed flow voids in both eyes.
The patient was sent to a location with indocyanine green angiography, which revealed a dense hypofluorescent lesion in the macula of each eye. This patient was diagnosed with persistent placoid maculopathy and now is treated with immunosuppression.
In patients with diabetic retinopathy, retina specialists care most about three things, according to Dr. Mammo:
“All of these factors affect your treatment recommendation,” he says. “OCT-A can help you evaluate these three things and make clinical decisions very quickly.”
For example, a 53-year-old man with well-controlled proliferative diabetic retinopathy presented with visual acuity of 20/80 in the right eye and 20/50 in the left eye. He claimed to have well-managed hemoglobin A1c and couldn’t understand why his vision was poor.
OCT-A produced clear images of enlarged foveal avascular zones that were affecting the patient’s vision. Despite adequate treatment of his diabetic macular edema and currently good control of his hemoglobin A1c, the patient was able to clearly see the impact of ischemia on his central vision when compared with a normal image.
Advertisement
“In situations like this, OCT-A can be a great teaching tool for patients,” says Dr. Mammo.
OCT-A also can help diagnose retinal neovascularization and ischemic burden, with or without correlating FA.
For example, a 30-year-old woman with proliferative diabetic retinopathy in both eyes deferred fluorescein testing due to pregnancy. OCT-A, especially with the wider 12 mm or 15 mm scans, was effective at showing severe ischemic burden.
Vitreoretinal interface slabs, while not clear, indicated possible lesions in her right and left eyes. By comparing the B-scan images, red flow revealed the presence of neovascular lesions in two instances, while lack of flow and improper segmented lines indicated segmentation error in a third instance.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/228dfc52-1738-4b8a-8398-64b9ddfebc9d/23-EYE-3929961-OCTA-in-DR-and-AMD-7)
Vitreoretinal interface slabs indicate possible neovascular lesions in the right eye of a 30-year-old patient with proliferative diabetic retinopathy.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b12f77cb-c17e-41ef-9d33-75b1641aafe2/23-EYE-3929961-OCTA-in-DR-and-AMD-8)
Red flow on a B-scan image reveals a neovascular lesion.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dbcd17b4-fc0e-4fee-b2f2-888da305095b/23-EYE-3929961-OCTA-in-DR-and-AMD-9)
Vitreoretinal interface slabs indicate possible neovascular lesions in the left eye of the same 30-year-old patient.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7fb1547c-7779-41ac-b7cc-8b83552142a3/23-EYE-3929961-OCTA-in-DR-and-AMD-10)
Correlating the B-scan image with the en face image (above) reveals a segmentation error, which created the false appearance of hyper-reflectivity in the vitreoretinal interface slab en face image — not a true neovascular lesion.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1b879ede-25cb-48e4-aaf7-95a70bb1c447/23-EYE-3929961-OCTA-in-DR-and-AMD-11)
En face imaging of the vitreoretinal interface slab of the same 30-year-old patient reveals a possible neovascular lesion superiorly.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/371e1c1a-207d-4ba6-bf64-2d8ab61c9c66/23-EYE-3929961-OCTA-in-DR-and-AMD-12)
Red flow on a B-scan image reveals a neovascular lesion that corresponds to the vitreoretinal interface slab (above).
The confirmation of clear neovascularization elsewhere confirmed a diagnosis of proliferative diabetic retinopathy.
In conclusion, for patients with diabetic retinopathy, OCT-A can be a great teaching tool, can quickly assess macular ischemia and has high sensitivity for diagnosing neovascularization.
Advertisement
New insights on effectiveness in patients previously treated with other anti-VEGF drugs
Evidence mounts that these diabetes and obesity drugs may protect eyes, not endanger them
CFH gene triggers the eye disease in white patients but not Black patients
A primer on sustained release options
Study explores association between sleep aid and eye disease
Early data show risk is 73% higher in patients with lupus, 40% higher in patients with rheumatoid arthritis
Switching medications may decrease treatment burden and macular fluid
Registry data highlight visual gains in patients with legal blindness