When and how to investigate further
By Jack Khouri, MD, Christy Samaras, DO, Jason Valent, MD, Alex Mejia Garcia, MD, Beth Faiman, PhD, CNP, Saveta Mathur, CNP, Kim Hamilton, CNP, Megan Nakashima, MD, and Matt Kalaycio, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The monoclonal gammopathies encompass a number of disorders characterized by the production of a monoclonal protein (M protein) by an abnormal clone of plasma cells or other lymphoid cells. Monoclonal gammopathy of undetermined significance (MGUS) is the most common of these disorders. The diagnostic criteria for MGUS are listed below.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9b2ae406-4f97-4a40-a19f-9cdbf9d8f482/19-ccr-009-table-1_550x1001_jpg)
Its clinical relevance lies in the inherent risk of progression to hematologic malignancies such as multiple myeloma or other lymphoproliferative disorders, or of organ dysfunction due to the toxic effects of the M protein. An M protein may consist of an intact immunoglobubin (Ig) molecule — i.e., two light chains and two heavy chains (most commonly IgG type followed by IgA and IgM) — or a light chain only (kappa or lambda).
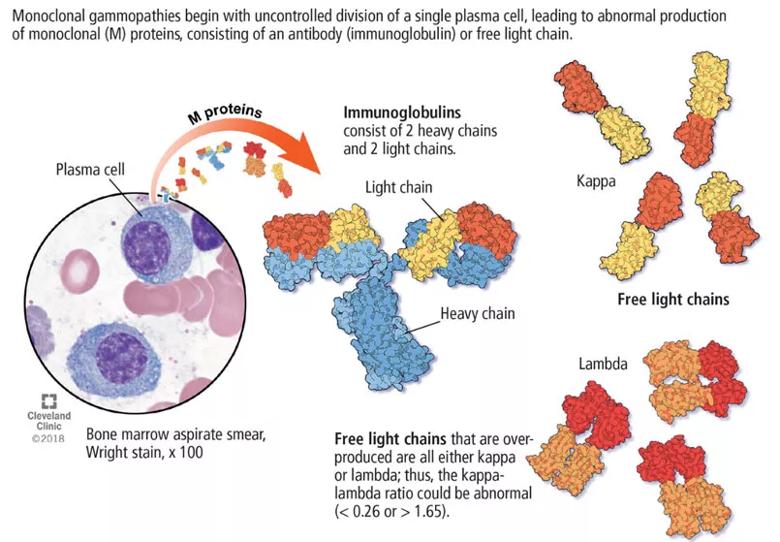
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3fece841-e062-4b21-8412-7c5ef52c9e74/19-ccr-009-image-1_805x559_jpg)
MGUS is present in 3 to 4 percent of the population over age 50 and is more common in older men, African-Americans and Africans.
The overall risk of progression to myeloma and related disorders is less than or equal to 1 percent per year depending on the subtype of the M protein (higher risk with IgM than non-IgM and light-chain MGUS). While the risk of malignant transformation is low, multiple myeloma is almost always preceded by the presence of an asymptomatic and often unrecognized monoclonal protein.
An M protein is typically an incidental finding when a patient is being assessed for any of a number of presenting symptoms or conditions. A large retrospective study found that screening for MGUS was mostly performed by internal medicine physicians. The indications for testing were anemia, bone-related issues, elevated creatinine, elevated erythrocyte sedimentation rate and neuropathy.
Advertisement
Routine screening for an M protein in the absence of clinical suspicion is not recommended, given the low risk of malignant progression, lack of effect on patient outcomes, the accompanying emotional burden and lack of treatment options. Evaluation for monoclonal gammopathy may be considered as part of the workup of associated clinical symptoms and signs and laboratory and imaging findings.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6e2d48ef-1c0c-49c8-bb2d-50c17c5f8437/19-ccr-009-table-2_550x800_jpg)
A low anion gap is not a major indicator of an M protein unless in a high concentration, in which case other manifestations would be present, such as renal failure, which would guide the diagnosis. Polyclonal hypergammaglobulinemia as a cause of low anion gap is far more common than MGUS.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/449b478c-a374-44fb-9e6d-28cc4927a26b/19-ccr-009-figure-2_550x1021_jpg)
Serum protein electrophoresis is an initial test used to identify an M protein and has a key role in quantifying it. An M protein appears as a narrow spike on the agarose gel and should be distinguished from the broad band seen in polyclonal gammopathies associated with cirrhosis and chronic infectious and inflammatory conditions, among others. A major disadvantage of serum protein electrophoresis is that it cannot detect an M protein in very low concentrations or determine its identity.
Serum immunofixation is more sensitive than serum protein electrophoresis and should always be ordered in conjunction with it, mostly to ensure detecting tiny amounts of M protein and to identify the type of its heavy chain and light-chain components.
The serum free light-chain assay is also considered an essential part of the screening process to detect light-chain MGUS and light-chain myeloma. As many as 16 percent of myeloma patients secrete only light chains, which may not be identified on serum immunofixation. In general, a low kappa-lambda ratio (< 0.26) indicates the overproduction of lambda light chains, and a high ratio (> 1.65) indicates the overproduction of kappa light chains.
Advertisement
The serum free light-chain assay helps detect abnormal secretion of monoclonal light chains before they appear in the urine once the kidney tubules become saturated and unable to reabsorb them.
Of note, the free light-chain ratio can be abnormal (< 0.26 or > 1.65) in chronic kidney disease. Thus, it may be challenging to discern whether an abnormal light-chain ratio is related to impaired light-chain clearance by the kidneys or to MGUS. In general, kappa light chains are more elevated than lambda light chains in chronic kidney disease, but the ratio should not be considerably skewed. A kappa-lambda ratio below 0.37 or above 3 is rarely seen in chronic kidney disease and should prompt workup for MGUS.
Tests in combination. The sensitivity of screening for M proteins ranges from 82 percent with serum protein electrophoresis alone to 93 percent with the addition of serum immunofixation and to 98 percent with the serum free light-chain assay. The latter can replace urine protein electrophoresis and immunofixation when screening for M protein, given its higher sensitivity. An important caveat is that urine dipstick testing does not detect urine light chains.
Once an M protein is found, immunoglobulin quantification, a complete blood cell count, and serum creatinine and calcium measurements are also recommended to look for anemia, renal failure and hypercalcemia, which can be associated with symptomatic myeloma.
Future posts will discuss the differential diagnosis of MGUS as well as risk stratification.
Advertisement
Dr. Khouri is a fellow in the Department of Hematology and Medical Oncology. Drs. Samaras, Valent and Mejia Garcia are staff in the Department of Hematology and Medical Oncology. Dr. Faiman, Ms. Mathur and Ms. Hamilton are clinical nurse practitioners in the Department of Hematologic Oncology and Blood Disorders. Dr. Nakashima is staff in the Department of Clinical Pathology. Dr. Kalaycio is Chairman of the Department of Hematology and Medical Oncology.
This abridged article was originally published in Cleveland Clinic Journal of Medicine.
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors