It’s more than inflammation
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
When IL-6 was discovered in the laboratory in the 1980s, it was considered a cytokine that primarily helped B cells in the process of making immunoglobulin. Since then, it has become clear that IL-6 is not only a key mediator in the inflammatory response, but also a link between the immune system and a variety of viscerosomatic tissues with far-reaching biology.
2017 was a big year for IL-6 biology with the approval of two new indications for tocilizumab, the lead compound in a class first approved to treat rheumatoid arthritis over a decade ago. This potent agent is now approved for inducing long-term, steroid-sparing remissions in giant cell arteritis and also for treatment of cytokine storm, which often accompanies the use of CAR-T cells, a major, recent breakthrough in the field of cancer immunotherapy.
I’ve worked with several collaborators including Stefan Rose-John, PhD, from University of Kiel and a pioneer in IL-6 biology and its signaling, as well as Ernie Choy, MD, Chair of Rheumatology at the University of Cardiff, and Kevin Winthrop, MD, of the University Of Oregon Health Sciences Center, on a series of reviews that discuss numerous recent observations pertinent to rheumatologists and immunologists who employ anti-IL-6-based strategies in their practice. I’ve highlighted the most compelling below.
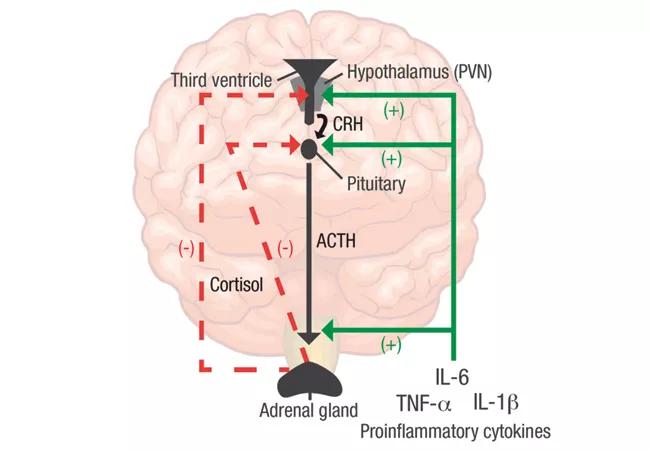
Recent work on the biology of IL-6 has demonstrated a key role in a wide variety of bodily systems including vascular, lipid metabolism, insulin resistance and neuropsychological behavior. IL-6 has also been demonstrated to be intimately involved in the interplay between the central nervous system and the immune system (see feature image). In rheumatoid arthritis (RA) and many other inflammatory conditions, there is dysregulation of the hypothalamic pituitary axis, and increasing evidence points to a critical role of inflammatory cytokines, especially IL-6. These effects likely belie the role of IL-6 in the domains of pain, fatigue and mood, which are critical to quality of life in patients with inflammatory diseases such as RA.
Advertisement
In terms of pain, preclinical models have suggested that IL-6 sensitizes animals to pain as glial cells and dorsal root ganglia express glycoprotein 130 and thus can be activated via trans-signaling with IL-6. In these experimental models of arthritis, IL-6 knockouts who are unable to respond to IL-6 significantly attenuate pain behavior. Clinical studies with biologics such as tocilizumab, sarilumab and sirukumab all support a potent capacity for IL-6 inhibition to mediate pain directly as well as via its key anti-inflammatory role in disease.
Fatigue is also a major comorbidity in numerous inflammatory conditions. Studies of normal volunteers infused with IL-6 demonstrated both disturbed sleep and fatigue. Fatigue in RA correlates with mood, pain and disability; thus, targeting IL-6 may be a useful strategy in approaching fatigue both directly and indirectly as a therapeutic strategy. It has been postulated that IL-6 may mediate this effect by directly disrupting the hypothalamic-pituitary axis which has been shown to be dysfunctional in patients with chronic fatigue syndrome.
Accordingly, it has become increasingly important to assess pain not only in clinical trials but also in the clinic. At Cleveland Clinic’s Department of Rheumatic and Immunologic Diseases, all patients are screened with quality of life measurements which center on the use of PROMIS® (Patient-Reported Outcomes Measurement Information System), a set of person-centered measures that evaluate and monitor physical, mental and social health in adults and children. To date, we have such measures on over 35,000 patient visits. Increasing evidence suggests enhanced patient engagement when they are able to see and reflect on issues that are import to their overall wellness, not merely the activity of their disease.
Advertisement
Finally, more than one-third of patients with RA have mood disorders, and depression ranks top among these. Numerous studies in animals and humans suggest a role for IL-6 in in the development of depression and anxiety. IL-6 is a sensitive biomarker in depression and in healthy individuals, and experimental administration of IL-6 significantly suppresses self-reported mood markers. In clinical trials, targeting of IL-6 in RA has been associated with significant improvements in mood scores and is superior to an anti-TNF comparator. Though the mechanisms involved are poorly understood, a clinical trial of the IL-6 receptor antagonist sirukumab for treatment refractory depression is ongoing and nearing completion.
The IL-6 space including its basic, translational and clinical immunology is rapidly changing, and numerous additional biologics are under development and soon may be available. Clinicians using these agents need to maintain a robust knowledge base in the field to better care for our patients. We highlighted some of these developments at the 6th Annual Basic and Clinical Immunology for the Busy Clinician, held March 9-10 in Miami, where over 120 clinical immunologists gathered for an immunology boot camp designed to strengthen skills in immunology as it relates to practice.
Dr. Calabrese directs the R.J. Fasenmyer Center for Clinical Immunology.
Feature image. The HPA axis in RA. Pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β stimulate cortisol and CRH release by acting at all three levels of the HPA axis (solid green lines). As a result, glucocorticoids regulate their own production through negative feedback on the upper levels of the HPA axis, including CRH in the PVN of the hypothalamus and ACTH in the anterior pituitary (dashed red lines). ACTH: adrenocorticotropic hormone; CRH: corticotropin-releasing hormone; PVN: paraventricular nucleus. Figure and caption republished with permission from Choy EHS, Calabrese LH. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology. 2017 Nov 22. doi: 10.1093/rheumatology/kex391. [Epub ahead of print].
Advertisement
Advertisement

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.

Unraveling the TNFA receptor 2/dendritic cell axis