Neuroimaging and next-generation sequencing help interrogate mimics
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Arriving at a diagnosis of central nervous system vasculitis (CNS-V) is fraught with challenges. Clinical presentation can be quite variable, and there is no classic presentation. Further complicating matters, the condition has several mimics, brain tissue is inaccessible and there is no disease-specific test. However, advances in neuroimaging and next-generation sequencing — along with the involvement of a multidisciplinary clinical team — have added formidably to our knowledge of CNS-V.
Regardless of the scenario, we take a systematic approach to the work-up of any patient suspected to have CNS-V. This approach includes a general history and physical exam, with a thorough review of symptoms associated with systemic autoimmune disease (e.g., fever, rashes, sinus disease, sicca, joint pains, cough, peripheral neuropathy, oro-genital ulcers, inflammatory eye disease, deep venous thrombosis or recurrent miscarriages). Clinicians should look for infectious and/or malignant conditions that might be associated with many of these nonspecific symptoms, especially fever, malaise, joint pains and weight loss. Other important aspects when interviewing patients include eliciting travel history, work hazards, chronic exposure to recreational drugs and family history of neurologic events; this information can unravel rare conditions.
Diagnosing CNS-V requires multiple modalities, to exclude other conditions, including autoimmune serology to rule out systemic disease, hypercoagulable profile, thoracic or transesophageal echocardiography, ECG and electrophysiology to rule out thrombotic/embolic conditions. Sampling cerebrospinal fluid is indispensable in ruling out mimics like infection or malignancy. Fluid biomarkers of CSF have been illusive, although recent investigations include IL-17, Th17, complement activation and Amyloid-beta A4 protein. Importantly, metagenomic next-generation sequencing for unbiased search for pathogens is quick, and is becoming less expensive. Such sequencing may reduce the need for expensive and risky testing, including brain biopsy.
Advertisement
Ocular imaging techniques can aid in the noninvasive diagnosis of CNS-V. Although primary CNS-V does not involve the ocular system, ocular imaging may help distinguish primary CNS-V from other mimics, such as sarcoidosis, Susac syndrome or primary CNS lymphoma.
High-resolution vessel wall MRI (HR-MRI) adds diagnostic value by showing distinguishing vessel wall patterns for CNS-V and other, noninflammatory diseases (e.g., reversible cerebral vasoconstriction syndromes). With HR-MRI, one can visualize the wall of large and medium intracranial vessels and may be able to distinguish between disparate vascular diseases with similar angiographic findings. HR-MRI has become part of imaging protocols detecting causes of ischemic stroke, mainly in a research setting but increasingly asked for (and used) in clinical practice; its precise role and added value for prognosis and patient care needs further elucidation.
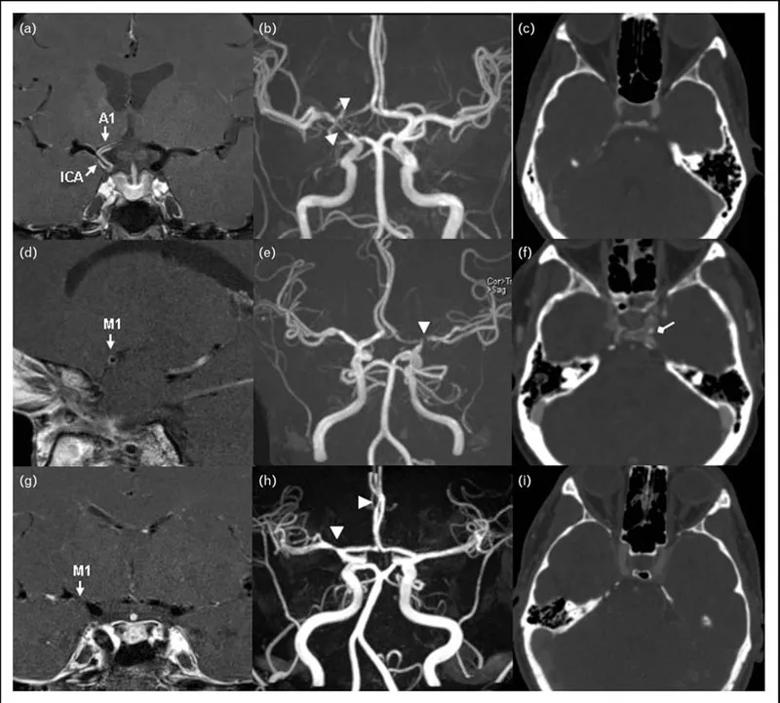
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d3b01a8e-c124-48d3-9fab-f788b88bc2c3/20-RHE-1861362-advDiagns-CNSvasculitis-HajjAli-inset_800x721_jpg)
Representative postgadolinium contrast high-resolution MRI vessel wall image (a, d, g), 3D-TOF magnetic resonance
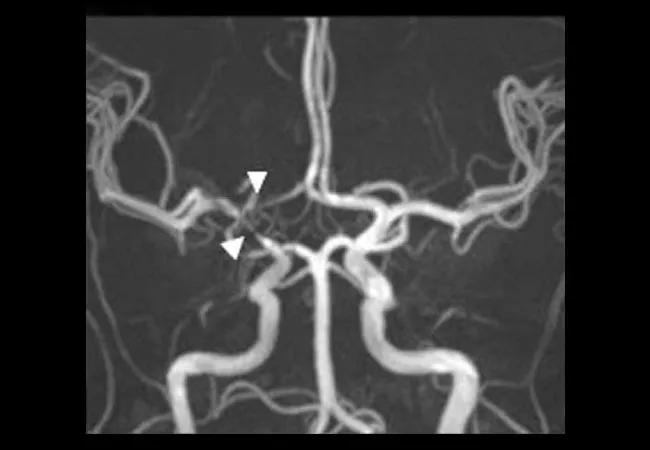
angiography (b, e, h), and contrast computed tomographic angiography (c, f, i) in patients with intracranial vasculopathy. (a, b, c) A 49-year-old female with central nervous system vasculitis. (a) T1-weighted arterial wall coronal image of the right terminal ICA and proximal A1 with strong smooth, concentric wall enhancement and thickening (arrows). (b) Magnetic resonance angiography revealed severe narrowing at the junction of the right terminal ICA, MCA, and ACA origin (arrow heads). (c) No evidence of intracranial ICA calcification. (d, e, f) A 47-year-old female with intracranial atherosclerotic disease. (d) T1-weighted arterial wall sagittal image showed mild wall thickening with eccentric wall enhancement at the left M1 segment (arrow). (e) Severe short segment stenosis was present at the origin of left MCA (arrow head). (f) Spotty calcification can be observed in the left intracranial ICA lumen (diamond arrow). (g, h, i) A 30-year-old female with reversible cerebral vasoconstriction syndromes. (g) T1-weighted arterial wall coronal image showed uniform wall thickening and wall narrowing without enhancement in the right M1 segment. (h) Magnetic resonance angiography revealed the mild to moderate narrowing in the proximal right MCA and the distal right ACA (arrow heads). (i) Intracranial ICA calcification was not present. 3D-TOF, 3D time of flight; ICA, intracranial artery; MCA, middle cerebral artery; ACA, anterior cerebral artery.
Advertisement
CNS-V can be potentially aggressive, making prompt, accurate diagnosis is important. Advances in molecular biology (e.g., next-generation sequencing) and neuroimaging add great value to the diagnostic dilemma; next-generation sequencing can enhance our ability to diagnose, interrogate and track infectious diseases and may help avoid brain biopsy in certain cases.
Note: Images reused with permission. Hajj-Ali R, Calabrese LH. Central nervous system vasculitis: advances in diagnosis. Curr Opinion Rheumatol. 2020 Jan;32(1):41-46.
Advertisement
Advertisement

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.

Unraveling the TNFA receptor 2/dendritic cell axis