Exploring links to depression, metabolic disease, cognitive impairment and more
By Harneet Walia, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
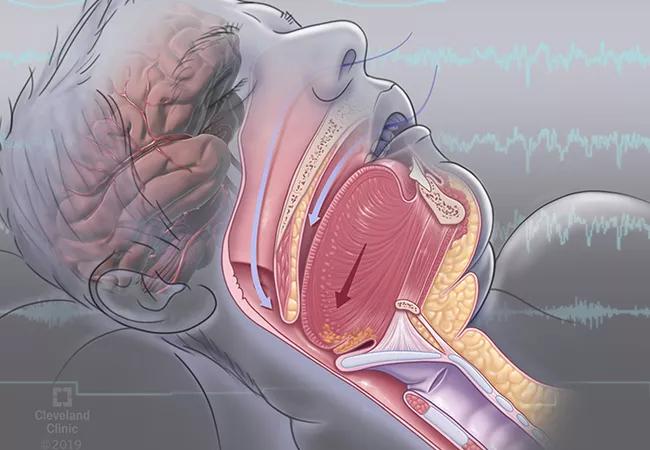
Obstructive sleep apnea (OSA) is a serious condition that impacts quality of life and causes drowsy driving and depression. Research also reveals an interrelationship between OSA and metabolic disease and an association between OSA and cognitive impairment.
The diagnostic criteria of OSA are based on the number of apneic or hypopneic episodes per hour of sleep, called the apnea–hypopnea index (AHI), as recorded during sleep testing. Diagnosis of OSA is warranted if the AHI is 15 or more per hour or if the AHI is five or more per hour with one or more of the following features:
Many patients with an AHI less than 15 also may have OSA, given the number of coexisting medical conditions included in the OSA diagnostic criteria. Heart conditions such as coronary artery disease, atrial fibrillation and congestive heart failure encompassed in the OSA diagnostic criteria have increased awareness of the link between OSA and heart health. Less well-known, and the subject of this review, are the negative consequences of OSA, particularly poor quality of life, drowsy driving, depression, metabolic disease and cognitive impairment.
Reduced quality of life is the most fundamental patient-reported outcome of OSA. OSA is associated with excessive daytime sleepiness, inattention and fatigue, which increase the risk of accidents and medical disability. These quality-of-life impairments are often the main reason patients seek medical care for sleep disorders.2 Improved quality of life is a central goal of OSA treatment and is the best indicator of the effectiveness of treatment.3 Sleep health and its effect on quality of life is an area of focus of Healthy People 2020.
Advertisement
The American Academy of Sleep Medicine identified quality of life, along with detection of disease and cardiovascular consequences, as an outcome measure for assessing the quality of care for adults with OSA.2 The assessment of quality of life for patients with OSA is a four-part process:
Information from these four processes can inform changes in a patient’s quality of life.
Treatment for OSA has been shown to improve quality of life. A study of 2,027 patients with OSA evaluated therapy adherence relative to mean Functional Outcomes of Sleep Questionnaire and European Quality of Life-5D scores.4 In patients with the most impaired quality of life, those adherent to positive airway pressure (PAP) therapy had improved quality of life as measured by these scores.
With respect to sleepiness, a systematic review of continuous PAP (CPAP) in patients with OSA found a 2.7-point reduction (mean difference; 95% confidence interval 3.45–1.96) in the Epworth Sleepiness Scale in patients using CPAP compared with the control group.5 Treatment of OSA improves patient quality of life and symptoms such as sleepiness.
Drowsy driving by people with OSA can lead to motor vehicle accidents, which result in economic and health burdens.6,7 National Center for Statistics and Analysis data reveal that of 6 million motor vehicle accidents (five-year average, 2005–2009), 1.4% involve drowsy driving and 2.5% of fatal crashes involve drowsy driving.8 Among noncommercial drivers, untreated OSA increases the risk of motor vehicle accidents 3- to 13-fold.9 The odds ratio of traffic accidents in drivers with untreated OSA is six times greater than in the general population.7
Advertisement
In a study of men and women in the general population (N = 913), individuals with moderate to severe OSA (AHI > 15) were more likely to have multiple motor vehicle accidents in the course of five years (odds ratio = 7.3) compared with those with no sleep-disordered breathing.10 The association between OSA and motor vehicle accidents is independent of sleepiness, and drivers with OSA may not perceive performance impairment.
There are two main reasons OSA increases the risk and incidence of motor vehicle accidents. OSA causes changes in attention and vigilance resulting from sleep deprivation and fragmentation. OSA also affects global cognition function, which may be due to intermittent hypoxia attributable to OSA.2
Treatment for OSA is effective in reducing the incidence of motor vehicle accidents. One study found the risk of motor vehicle accidents was eliminated with the use of CPAP treatment in patients with OSA.11 A recent study of nearly 2,000 patients with OSA found a reduction in self-reported near-accidents from 14% before PAP therapy to 5.3% after starting PAP therapy.12
Depression can occur as a consequence of OSA. Moreover, depression and OSA have several symptoms in common, including daytime sleepiness, fatigue, loss of energy, poor concentration, irritability, psychomotor retardation and weight gain.13
Estimates of the prevalence of depression in patients with OSA range from 5% to 63%.13,14 One year after patients were diagnosed with OSA, the incidence of depression per 1,000 person-years was 18% compared with 8% in a group without OSA.14 Women with OSA reportedly have a higher risk of depression (adjusted hazard ratio [HR] 2.7) than men (HR 1.81) at 1-year follow-up.14 In the same study, with respect to age, there was no significant relationship noted between OSA and patients over age 64.
Advertisement
A one-level increase in the severity of OSA (i.e., from minimal to mild) is associated with a nearly twofold increase in the adjusted odds for depression.15 On the other hand, studies have also found that patients on antidepressants may have an increased risk of OSA.16
Several potential mechanisms have been proposed to explain the link between depression and OSA.13 Poor-quality sleep, frequent arousal and fragmentation of sleep in OSA may lead to frontal lobe emotional modulation changes. Intermittent hypoxia in OSA may result in neuronal injury and disruption of noradrenergic and dopaminergic pathways. Pro-inflammatory substances such as interleukin 6 and interleukin 1 are increased in OSA and depression, and are mediators between both conditions. Neurotransmitters may be affected by a disrupted sleep-wake cycle. And serotonin, which may be impeded in depression, could influence the upper-airway dilator motor neurons.
Treatment of OSA improves symptoms of depression as measured by the Patient Health Questionnaire (PHQ-9). After three months of compliance with CPAP therapy, mean PHQ-9 scores decreased from 11.3 to 2.7 in a study of 228 patients with OSA.17 A study of 1,981 patients with sleep-disordered breathing found improved PHQ-9 scores in patients compliant with CPAP therapy and a greater improvement in patients with a baseline PHQ-9 higher than 10 (moderate severity).18
OSA is associated with metabolic disorders, including metabolic syndrome, though the causality between these two conditions is yet to be illuminated. Metabolic syndrome is a term used when an individual has three or more of the following features or conditions:
Advertisement
Metabolic syndrome increases an individual’s risk of diabetes and cardiovascular disease and overall mortality. Like OSA, the prevalence of metabolic syndrome increases with age in both men and women.20,21 The risk of metabolic syndrome is greater with more severe OSA. The Wisconsin Sleep Cohort (N = 546) reported an odds ratio for having metabolic syndrome of 2.5 for patients with mild OSA and 2.2 for patients with moderate or severe OSA.22 A meta-analysis also found a 2.4 times higher odds of metabolic syndrome in patients with mild OSA, but a 3.5 times higher odds of metabolic syndrome in patients with moderate to severe OSA compared with the control group.23
Patients with both OSA and metabolic syndrome are said to have syndrome Z24 and are at increased risk of cardiovascular morbidity and mortality.25 Syndrome Z imparts a higher risk of atherogenic burden and prevalence of atheroma compared with patients with either condition alone.26 In comparing patients with metabolic syndrome with and without OSA, those with OSA had increased atherosclerotic burden as measured by intima-media thickness and carotid femoral pulse-wave velocity.27 Syndrome Z is also linked to intracoronary stenosis related to changes in cardiac morphology28 and is associated with left ventricular hypertrophy and diastolic dysfunction.29
OSA and hypertension. Hypertension is one of the conditions encompassed in metabolic syndrome. Several studies report increased risk and incidence of hypertension in patients with OSA. In a community-based study of 6,123 individuals age 40 and older, sleep-disordered breathing was associated with hypertension, and the odds ratio of hypertension was greater in individuals with more severe sleep apnea.30 Similarly, a landmark prospective, population-based study of 709 individuals over four years reported a dose-response relationship between patients with OSA and newly diagnosed hypertension independent of confounding factors.31 Patients with moderate to severe OSA had an odds ratio of 2.89 of developing hypertension after adjusting for confounding variables.
A study of 1,889 individuals followed for 12 years found a dose-response relationship based on OSA severity for developing hypertension.32 This study also assessed the incidence of hypertension based on CPAP use. Patients with poor adherence to CPAP use had an 80% increased incidence of hypertension, whereas patients adhering to CPAP use had a 30% decrease in the incidence of hypertension.
Resistant hypertension (i.e., uncontrolled hypertension despite use of three or more antihypertensive and diuretic medications) has been shown to be highly prevalent (85%) in patients with severe OSA.33 An analysis of patients at increased risk of cardiovascular disease and untreated severe OSA was associated with a four times higher risk of elevated blood pressure despite intensive medical therapy.34
Mechanisms of altered metabolic regulation in OSA. Mechanisms implicated in altering metabolic regulation in OSA include intermittent hypoxia, sleep fragmentation and glucose homeostasis, and obesity. Intermittent hypoxia from OSA results in sympathetic nervous system activation that affects the pancreas, skeletal muscle, liver and fat cells, resulting in altered insulin secretion, lipid-bile synthesis, glucose metabolism and lipoprotein metabolism.35
Sleep fragmentation is a cardinal feature of OSA, and the resulting suppression of sleep may alter insulin sensitivity. Studies have implicated disruptions to slow-wave sleep specifically, as well as disruption of any stage of sleep in reduced insulin sensitivity.35,36 In addition to decreased insulin sensitivity, sleep fragmentation also increases morning cortisol levels and increases sympathetic nervous system activation.37
Obesity and OSA share a pathway imparting increased cardiometabolic risk.38 Fat tissue causes higher systemic inflammation and inflammatory markers. A recent report describes a bidirectional relationship between metabolic syndrome and OSA.39 While OSA increases the risk for metabolic syndrome, metabolic syndrome by virtue of body mass index with changes in mechanical load and narrow airway and physiology can predispose for OSA.
Effect of treatment for OSA on metabolic syndrome. Several studies have evaluated the effect of CPAP treatment for OSA on metabolic syndrome overall, as well as the specific conditions that comprise metabolic syndrome. In evaluating CPAP use and metabolic syndrome overall, studies have found a reduced prevalence of metabolic syndrome,40,41 CPAP benefit only in complying patients,42 and a reduction in oxidative stress with a single-night use of CPAP.43
With respect to insulin sensitivity, a study of 40 men with moderate OSA using CPAP therapy (mean use five hours) reported an increase in the insulin sensitivity index after two days, and a further increase after three months.44 Another study found no improvement in insulin resistance in severe OSA.45 A meta-analysis reported improved insulin resistance with CPAP,46 although a recent meta-analysis assessing hemoglobin A1c level, fasting insulin level and fasting glucose did not show improvement in these parameters. Large-scale clinical trials with longer treatment duration and better CPAP compliance are warranted.47
CPAP use in patients with OSA has been found to affect hypertension in a number of studies.48-55 In a comparison of therapeutic CPAP with suboptimal CPAP for nine weeks, ambulatory blood pressure was reduced in the therapeutic group and no change was seen in the subtherapeutic group, illustrating the importance of optimal pressure settings in treating OSA.48
A randomized controlled trial of nearly 300 individuals found improvement in six blood pressure parameters in a group using CPAP compared with a group using sham CPAP after 12 weeks.50 A large clinic-based cohort of 894 individuals with hypertension and resistant hypertension (15%) found that after one year, CPAP use was associated with 2-3 mm Hg of reduction in blood pressure.56 Meta-analysis of randomized controlled trials on the effectiveness of CPAP on hypertension found reductions of 2-3 mm Hg in blood pressure.57 Another meta-analyses showed a reduction of 2.6 mm Hg in 24-hour mean blood pressure with CPAP therapy.48-55 This reduction may appear modest in nature; however, any reduction in blood pressure can result in decreased cardiovascular morbidity and mortality. A meta-analysis of randomized controlled trials indicated reductions in mean systolic blood pressure of 5.4 mm Hg and diastolic blood pressure of 3.86 mm Hg after CPAP in those with resistant hypertension and OSA.58
Weight loss has been shown to reduce the AHI and other parameters related to sleep apnea, such as oxygen desaturation index in patients with obesity and diabetes.59 Weight loss combined with CPAP compared with CPAP or weight loss alone showed an incremental benefit in improving glucose parameters, triglycerides and possibly systolic blood pressure and triglycerides.60
Data suggest that OSA is linked with cognitive impairment, may advance cognitive decline, and is a bidirectional relationship. Women with OSA were reportedly more likely to develop mild cognitive impairment compared with women without OSA.61 An elevated oxygen desaturation index and a high percentage of time spent with hypoxia was associated with increased risk of developing mild cognitive impairment and dementia.
OSA was found to be an independent risk factor for cerebral white matter changes in middle-age and older individuals. Moderate to severe OSA imparted a two times higher risk of cerebral white matter changes compared with individuals without OSA.62 Another study of 20 patients with severe OSA compared with 40 healthy volunteers found diffusion imaging consistent with impaired fibrin integrity in those with OSA, indicative of white matter microstructure damage. The impairment was associated with increased disease severity and higher systemic inflammation.63
Individuals with hypoxia for a high percentage of time during sleep had a four times higher odds of cerebral microinfarcts.64 Cognitive scores decreased less in men. Men typically have more time in slow-wave sleep, suggesting that slow-wave sleep may be protective against cognitive decline. Mild cognitive impairment and Alzheimer disease were found more likely to develop and occur at an earlier age in individuals with sleep-disordered breathing compared with individuals without sleep-disordered breathing.65
OSA was also associated with increased serum amyloid beta levels in a study of 45 cognitively normal patients with OSA compared with 49 age- and sex-matched control patients. Increased amyloid beta levels correlated with increasing severity of sleep apnea as measured by the AHI.66
Mechanism linking OSA and cognition. One possible mechanism linking sleep quality and cognitive impairment or Alzheimer disease is the role of unfragmented sleep in attenuating the apolipoprotein E e4 allele on the incidence of Alzheimer disease.67 Beta amyloid is released during synaptic activity. Neuronal and synaptic activity decreases during sleep, and disrupted sleep could increase beta amyloid release.68 Sleep has been found to enhance the clearance of beta amyloid peptide from the brain interstitial fluid in a mice model.69
Recent data point toward the bidirectional relationship between the sleep and Alzheimer disease in that excessive and prolonged neuronal activity in the absence of appropriately structured sleep may be the reason for both Alzheimer disease and OSA.70,71
Effect of treatment for OSA on cognition. White matter integrity in 15 patients with OSA before and after treatment with CPAP was compared with 15 matched controls. Over 12 months, there was a nearly complete reversal of white matter abnormalities in patients on CPAP therapy.72 Improvement in memory, attention and executive function paralleled the changes in white matter after the treatment.
OSA is a serious condition with far-reaching consequences associated with impaired quality of life, depressive symptoms, drowsy driving, metabolic disease and cognitive decline. Treatment of OSA improves many of these health consequences, emphasizing the need for OSA screening. Large randomized studies are needed to assess the efficacy of CPAP on metabolic outcomes, including insulin sensitivity and glucose tolerance, in reducing disease burden. Study of the endophenotypes of patients with OSA is needed to define and target the mechanisms of OSA-induced adverse health outcomes and perhaps lead to personalized care for patients with OSA.
Note: This post is a slight adaptation of an article published in Cleveland Clinic Journal of Medicine (2019 September;86(9 suppl 1):19-25).
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help