Both procedures required unconventional approaches
Cleveland Clinic surgical teams led by Intestinal Transplant Program Co-Director Anil Vaidya, MD, recently performed two highly complex intestinal transplants, each a first of its kind.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
One case involved a multivisceral transplant to treat life-threatening complications from a rare appendiceal cancer; the other was an isolated small bowel transplant for dysmotility that preserved the patient’s previously constructed Indiana Pouch.
Under the leadership of Dr. Vaidya and Kareem Abu-Elmagd, MD, PhD, Cleveland Clinic’s intestinal transplant program was the nation’s most active in 2021, performing almost 20% of the 96 surgeries that took place.
“The two novel transplants’ success is due to the program’s advanced capabilities and experience, collaboration across specialties, and the willingness to push boundaries to achieve the best outcome for patients,” Dr. Vaidya says.
“Intestinal transplants cannot be an isolated activity,” he says. “These procedures were team-of-teams efforts. Cleveland Clinic is the place to do these complex operations because of the experts and resources available here. The attitude is always, ‘We can do it.’”
A 32-year-old man with a four-year history of intermittent abdominal pain, bloating and early satiety and a 2019 diagnosis of pseudomyxoma peritonei (PMP) was referred to Cleveland Clinic in 2021 in the end stage of his disease.
PMP is an uncommon abdominal malignancy arising from a perforated polyp, usually in the appendix. The lesion’s rupture produces free-floating, mucous-secreting epithelial cells that distribute widely in the abdominal cavity, eventually concentrating, implanting and forming mucinous tumors on peritoneal surfaces, particularly the greater and lesser omentum and the underside of the diaphragm. Slow but progressive tumor enlargement causes fistulas, strictures and obstructions that impair gastrointestinal function. Malnutrition and life-threatening complications ultimately occur.
Advertisement
Treatment traditionally involves extensive cytoreductive surgery (CRS) to resect macroscopic tumors paired with hyperthermic intraperitoneal chemotherapy (HIPEC) to eradicate microscopic cancer cells. The treatment’s goal is to completely eradicate abdominal tumors or, less optimally, to debulk them while also stripping as much of the peritoneal lining as possible to reduce the potential for additional neoplasms.
While the CRS/HIPEC combination has significantly improved PMP patients’ survival and can be curative, disease recurs in roughly 20% to 30% of patients within 10 years. Approximately half of those patients’ recurring tumors are unresectable due to extensive bowel involvement.
Historically, active cancer had been considered a contraindication for solid organ transplantation, with the rationale that recurrence involving or affecting the donor organ would constitute misuse of a scarce resource.
During the last two decades, liver transplantation to treat hepatobiliary cancers and liver-only metastasis of nonhepatobiliary carcinomas has emerged as a viable therapeutic approach, inaugurating the field of transplant oncology.
In 2013, as the leader of Oxford (UK) University Hospitals’ intestinal transplant program, Dr. Vaidya extended the concept of transplant oncology to intestinal transplantation by performing the world’s first modified multivisceral transplant (including stomach, pancreaticoduodenal complex, small bowel, colon and abdominal wall) to treat a patient with unresectable PMP.
Advertisement
Although the Oxford hospitals’ institutional review board had approved the procedure’s use in this experimental circumstance, some peritoneal oncologists were critical because of concerns of tumor recurrence.
Dr. Vaidya was confident that completely excising the secretory peritoneum as part of transplantation — which is not possible with CRS — would eliminate the source for ongoing tumorigenesis. “I was heavily criticized” for undertaking the surgery, he says. “It was a leap of faith for some people to believe that an intestinal transplant could be effective for a condition that was considered untreatable” in its later stage.
The initial transplant patient succumbed to graft-versus-host disease and associated sepsis, and a subsequent patient died following an anastomotic leak and gastrointestinal hemorrhage. But a total of 15 PMP patients transplanted in the Oxford program have survived and remained cancer-free for a mean of five years, Dr. Vaidya reports.
The transplants’ success prompted physicians at the UK’s Peritoneal Malignancy Institute to alter their PMP treatment protocol, according to Dr. Vaidya. Rather than performing upfront CRS and HIPEC, patients are directly referred for multivisceral transplant. “We did the first case in the world of a de novo transplant, without any debulking,” Dr. Vaidya says. “It is changing the way this disease is being seen,”
In the United States, however, CRS/HIPEC remains the initial therapy for PMP.
The patient referred to Dr. Vaidya at Cleveland Clinic in 2021 previously had undergone surgical debulking of multiple intra-abdominal tumors, along with argon beam ablation, HIPEC, splenectomy, omentectomy, peritonectomy, cholecystectomy, appendectomy, biliary sphincterotomy and common bile duct stenting. Bulky disease remained at the hepatic hilum, epigastrium, pancreas, ascending colon, transverse colon, descending colon, sigmoid colon and rectum.
Advertisement
His tumor burden increased, with subsequent complications including portal vein thrombosis, gastric outlet obstruction, encasement of the duodenum, tumor incursion into the bladder, severe segmental narrowing of the proximal sigmoid colon, and bowel perforation with bacterial peritonitis and developing cholangitis. The patient was started on total parenteral nutrition and a gastrojejunostomy tube was placed for feeding and venting. Stents were placed in the duodenum and colon but developed perforations and abscesses.
At the time of referral, the patient was receiving hospice care.
“We needed to perform an evaluation to determine if transplantation in his case was safe, feasible and could provide long-term benefits,” Dr. Vaidya says.
Imaging showed that a tumor had encased the patient’s liver, compromising its blood supply. Had Dr. Vaidya seen the patient in his Oxford practice, that complication would have precluded a transplant, since UK organ procurement organizations resisted including a liver with the other donor organs for what was considered an experimental procedure.
At Oxford, “my criteria for transplantation for PMP was someone who did not have liver involvement, because getting a liver as part of the donor graft was not possible,” Dr. Vaidya says. The transplants he had performed to treat PMP patients were modified multivisceral procedures, excluding the liver.
In this case, the tumor affecting the patient’s liver was advanced but still contained within the abdominal cavity, with no sign of metastasis. The evaluation determined that a multivisceral transplant was justified. Dr. Vaidya received approval from Cleveland Clinic’s Intestinal Transplant Selection Committee to proceed and was able to place the patient on the national transplant wait list in mid-2021.
Advertisement
Compatible organs became available in September 2021. Immediately prior to surgery, the patient received induction therapy with the monoclonal antibody alemtuzumab to reduce the risk of acute rejection.
The multivisceral transplant took place over 17 hours and involved seven transplant, colorectal and urologic surgeons.
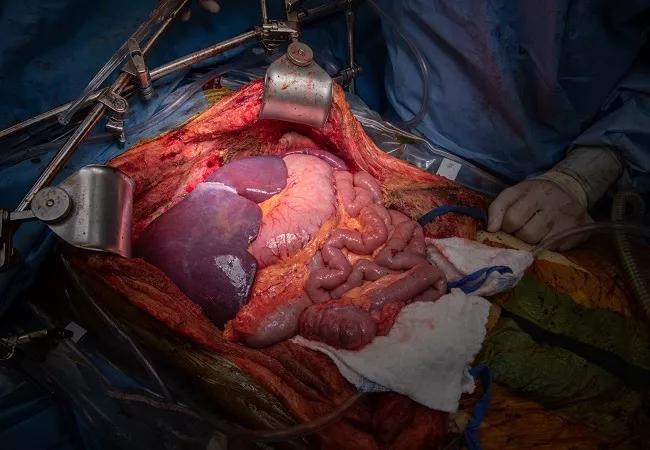
Despite previous debulking, extensive intra-abdominal tumors remained, including one encasing the patient's liver and another invading the bladder.
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c0f14789-77c8-47b9-ba09-4923f002e45c/22-DDI-2681609-Multivisceral-tx-case-reports-slideshow-1-800x550-1-150x100_jpg)
Surgeons carefully resect the patient's diseased organs.
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d6d1a1b6-4027-48b4-aa01-ad75514a1ca2/22-DDI-2681609-Multivisceral-tx-case-reports-slideshow-2-800x550-1-150x100_jpg)
Preparing the donor organs for transplantation.
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/70cf5bef-109d-4c56-9e50-7321f1d830a6/22-DDI-2681609-Multivisceral-tx-case-reports-slideshow-3-800x550-1-150x100_jpg)
Removal of the native organs.
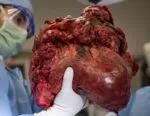
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4f02b9a0-0bce-47fd-9b57-eb798815232b/22-DDI-2681609-Multivisceral-tx-case-reports-slideshow-4-800x550-1-150x116_jpg)
Cleveland Clinic Intestinal Transplant Program Co-Director Anil Vaidya, MD, (right, wearing black and blue surgical cap) and transplant surgeon Masato Fujiki, MD, at work during the multivisceral transplant.
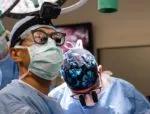
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9ee58023-70ab-4ead-8fa5-bd3cf2bfa650/22-DDI-2681609-Multivisceral-tx-case-reports-slideshow-7-150x114_jpg)
Preparing for implantation and anastomosis of the donor organs.
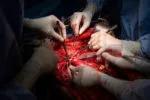
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b321812a-50c7-45d8-83a5-ebf189ad0537/22-DDI-2681609-Multivisceral-tx-case-reports-slideshow-5-800x550-1-150x100_jpg)
The multivisceral graft after reperfusion and hemostasis are achieved.
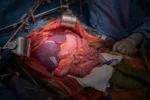
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8c6c3c1b-bb85-49a6-9c13-5cce923d1cee/22-DDI-2681609-Multivisceral-tx-case-reports-slideshow-6-150x100_jpg)
At the outset, the urology team placed bilateral ureteral stents to protect the kidneys and ureters. Dr. Vaidya began by incising the abdomen, which was filled with a mixture of dense adhesions from the patient’s previous surgery and the solidified components of the mucus-secreting tumor pathology.
The transplant’s preparatory phase required more than three hours to complete and included dissection, tumor debulking, mobilization of the native organs and their vasculature, removal of the pre-existing duodenal and colonic stents, takedown of the venting gastrostomy, left colectomy, and excision of the bladder tumor and partial cystectomy.
The native abdominal organs were then removed and Dr. Vaidya constructed a jump graft on the infrarenal aorta using donor aorta to supply arterial inflow to the multivisceral graft.
The donor organs — liver, stomach, pancreaticoduodenal complex, spleen, small intestine and right colon — were placed in the abdomen and their vascular inflow and outflow connections established. The multivisceral graft was reperfused and hemostasis was achieved.
The spleen was temporarily transplanted and reperfused to provide immunologic protection of the other transplanted organs and to facilitate blood flow to the donor pancreas until it could be reperfused. This also provided a vascular advantage of keeping the splenic vein open. The right colon was temporarily transplanted to allow the recipient’s cells to mix with the donor microbiota and identify their molecular signature, which could prevent bacterial overgrowth and infectious complications post-transplant. Both the donor spleen and right colon were removed prior to completion of the transplant since their purpose aiding the viability of the transplanted organs had been served.
The colorectal and transplant surgical teams made functional connections by performing a native esophago/transplant gastrostomy and graft cholecystectomy, splenectomy, hemicolectomy and pyloroplasty. They created a left-sided end ileostomy. Finally, the abdominal cavity was washed with warm saline, the fascia was closed with biologic mesh and the abdominal skin incision was stapled.
The operation was the world’s first full multivisceral transplant to treat PMP.
The patient’s initial postoperative course was difficult. He developed symptoms of infection, was treated with antibiotics and antifungals, and underwent an exploratory laparotomy 10 days after the transplant for lysis of adhesions, fluid drainage and abdominal washout. After aspirating bile, the patient developed a respiratory infection, pleural effusion and acute respiratory distress syndrome that required multiple days of intubation and medical treatment.
“The ICU team was fantastic,” Dr. Vaidya says. The patient’s condition improved. “He started turning the corner around three weeks after the transplant. He began eating and drinking and the stoma was working.”
The patient was discharged but approximately seven weeks post-transplant he was rehospitalized for a skin rash indicative of graft-versus-host disease (GVHD). Symptoms progressed despite steroid treatment, so colorectal surgeon Amy Lightner, MD, Director of Cleveland Clinic’s Center for Regenerative Medicine and Surgery, administered three doses of mesenchymal stromal cell (MSC)-derived exosomes — another first in a post-multivisceral transplant setting.
MSCs exert a powerful immunomodulatory effect and are able to reverse the progression of GVHD. Exosomes, or extracellular vesicles, isolated from MSCs possess the targeting capabilities of their parent cells but are not highly immunogenic and lack malignant potential and other inherent the risks of cell-based therapies, making them ideal to restore micromolecular homeostasis.
Exosomal treatment for GVHD “is a novel, first-ever treatment in solid organ transplants,” says Dr. Vaidya. “Dr. Lightner obtained an emergency use authorization from the Food and Drug Administration to treat the patient. His recovery was absolutely startling. His symptoms abated within two hours of the first dose.”
The patient has resumed a normal diet and lifestyle. He takes an immunosuppressant and a steroid to prevent rejection and is monitored with periodic endoscopy.
A 35-year-old woman with dysmotility and a history of interstitial cystitis was referred to Cleveland Clinic in 2021 for evaluation for isolated intestine transplant.
The patient had experienced chronic abdominopelvic pain, nausea, bloating and constipation since childhood. She underwent several surgeries as a young adult, including laparoscopic cholecystectomy, laparoscopic lysis of endometriosis adhesions, appendectomy and removal of an endometrial implant on her bladder.
In 2016, after nonsurgical interstitial cystitis treatments proved unsuccessful, the patient underwent a simple cystectomy with creation of an Indiana Pouch — a continent, catheterizable ileocecal reservoir for urinary diversion. In the next several years, her nausea, vomiting and constipation worsened in spite of maximal medical therapy. She underwent exploratory laparoscopy, pyloroplasty, placement of a gastrojejunostomy tube and, in 2020, a loop ileostomy and parastomal hernia repair. After developing dysmotility and irreversible intestinal failure, she began total parenteral nutrition. Ultimately, she was advised to consider intestinal transplantation.
Circulatory inflow for the patient’s Indiana Pouch was supplied by the right colic artery (RCA), branching from the superior mesenteric artery (SMA). In a typical isolated intestinal transplant procedure, the native SMA is transected proximal to the RCA juncture to enable exenteration of the small intestine. According to several intestinal transplant programs the patient consulted, preserving the Indiana Pouch’s vasculature might compromise circulation to the transplanted intestine or pose overly challenging dissection problems. The alternative — removing the Indiana Pouch and constructing a replacement urinary diversion using a portion of the donor bowel — could make the new pouch vulnerable in the event of a severe rejection episode.
While at Oxford, Dr. Vaidya had performed Indiana Pouch-sparing and ileal conduit-sparing intestinal transplants, but there are no reports of the procedures having been done in the United States.
“The majority of transplant centers don’t have the thought process of saving some native organs and transplanting only what you need to,” Dr. Vaidya says. After viewing CT scans that delineated the intestinal vasculature, “I was the first to say I think we can preserve the Indiana Pouch. With my background in urology, I could visualize the pouch’s blood supply and where the ureters enter. I could visualize separating it from the intestine. The evaluation process was of paramount importance. We looked at all the scans, and the blood supply to the Indiana Pouch was clearly distinct. And I knew I could stay away from that.”
In the event that the blood supply to the patient’s Indiana Pouch could not be preserved, the transplant team planned to create an ileal conduit from a segment of the donor bowel. Unlike the Indiana Pouch, an ileal conduit is not continent and requires an external collection pouch.
The isolated intestinal transplant took place over 10.5 hours and involved six transplant and colorectal surgeons.
The procedure began with extensive lysis of abdominal adhesions so the surgeons could visualize the Indiana Pouch and its ureters and preserve the pouch’s vascular pedicle. The patient’s gastrostomy and loop ileostomy were taken down.
The surgeons then removed the patient’s diseased native small bowel and colon, resecting close to the intestine’s antimesenteric border to help preserve any collateral blood vessels supplying the Indiana Pouch.
To prepare for the vascular connections to the intestinal allograft, transplant surgeon Mohammed Osman, MD, dissected areas on the patient’s infrarenal aorta and inferior vena cava (IVC). He also constructed an arterial jump graft using a segment of donor iliac artery.
Working with the allograft, transplant surgeon Masato Fujiki, MD, identified the superior mesenteric vein (SMV) and superior mesenteric artery (SMA). He ligated and cut the small vessels in the root of the mesentery to lengthen the vessels. He identified and ligated the splenic extension from the SMV.
The intestinal allograft was positioned in the recipient’s abdomen and the donor SMV was anastomosed to the native IVC in an end-to-side fashion. The donor SMA was anastomosed to the previously constructed arterial jump graft from the native infrarenal aorta.
Clamps were released and reperfusion was uneventful. The allograft perfused well throughout its length.
Dr. Lightner then constructed a gastro-jejunostomy to connect the native stomach to the allograft. She anastomosed the remnant limb of the native biliary-pancreatic complex to the transplant jejunum approximately 35 centimeters away from the gastro-jejunostomy to avoid reflux of bile into the efferent limb.
The surgical team had considered connecting the allograft to the native rectum but elected not to proceed, based on the appearance of the pelvic floor and presurgical results of rectal manometry.
A terminal ileostomy was brought out of the previous loop ileostomy site. A parastomal hernia in the loop ileostomy site was dissected out and repaired. An end ileostomy was fashioned and matured. The patient’s anterior abdominal wall was reconstructed using biologic mesh sutured to the rectus sheath and the abdominal incision was closed.
After an uneventful recovery and discharge several weeks following the transplant, the patient has resumed a regular diet. “She is exploring new foods,” Dr. Vaidya says. “The joy that she’s gotten back into her life is spectacular to see.”
Advertisement

Multidisciplinary framework ensures safe weight loss, prevents sarcopenia and enhances adherence

Study reveals key differences between antibiotics, but treatment decisions should still consider patient factors

Key points highlight the critical role of surveillance, as well as opportunities for further advancement in genetic counseling

Potentially cost-effective addition to standard GERD management in post-transplant patients

Findings could help clinicians make more informed decisions about medication recommendations

Insights from Dr. de Buck on his background, colorectal surgery and the future of IBD care

Retrospective analysis looks at data from more than 5000 patients across 40 years

Surgical intervention linked to increased lifespan and reduced complications