A quick review of 3D-printed models, intrasaccular flow disruption and flow diverter stenting
“It’s a great time to be a brain aneurysm surgeon,” says Mark Bain, MD, MS, Head of Cerebrovascular and Endovascular Neurosurgery at Cleveland Clinic. “We used to have a couple of options. And now, everyday, we’re hearing about a new device that’s on the horizon.”
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Dr. Bain discussed the latest breakthroughs in brain aneurysm treatment in his recent Neuro Pathways podcast.
“Now about 90% of aneurysms are treated from inside the blood vessel,” he says. “Patients are going home with a little bandage on their groin or on their wrist, and they’re getting back to work in two days.”
Reducing the morbidity of treatment and returning patients to their regular lifestyle is the main goal, he says.
Here’s a quick review of the latest advances that are helping Dr. Bain and other neurosurgeons improve outcomes for patients with brain aneurysms.
In April 2019, clinicians in Cleveland Clinic’s Cerebrovascular Center performed one of the first reported brain aneurysm repairs guided by a 3D-printed replica of the aneurysm. The model was produced at actual size, based on angiograms.
“The 3D-printed model allowed me to visualize the surgical approach before I made a single incision, enabling me to select the clip size in advance and know the exact location of the important daughter branches coming out of the aneurysm,” says Dr. Bain.
In addition to improving operative planning, 3D-printed replicas may help:
The technology can replicate some of the smallest aneurysms, notes Dr. Bain. Some branches in this first model were under 1 mm but still clearly visible.
Dr. Bain’s team is pursuing grant funding to continue using 3D-printed replicas, with the intent of accumulating enough data to assess outcomes and overall costs.
Advertisement
A new type of device promises to change the treatment of wide-neck bifurcation aneurysms.
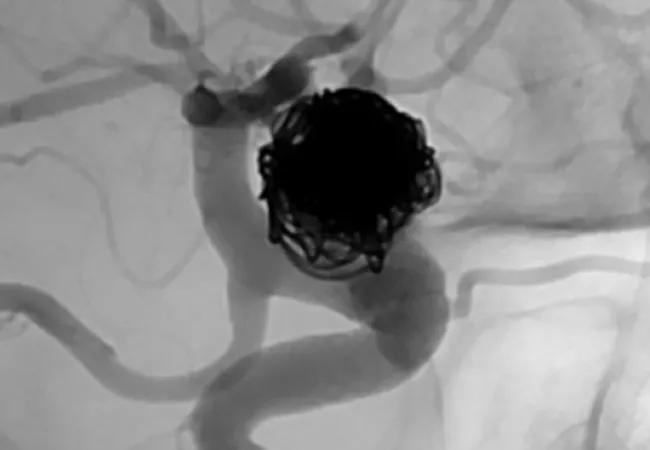
In late 2018, the FDA announced premarket approval (PMA) of the Woven EndoBridge (WEB) Aneurysm Embolization System from MicroVention, Inc. This was the first U.S. PMA of an intrasaccular flow disruptor for aneurysm embolization. The device has been used in Europe since 2010.
The WEB System consists of a permanent nickel/titanium self-expanding mesh ball implant along with a delivery wire and controller. The implant comes in two different shapes and various sizes to match to the aneurysm size. It is inserted in the groin and delivered by endovascular route to the intracranial aneurysm sac, where it deploys and fills the aneurysm. The mesh provides tension so the device remains in place, disrupting blood flow to the aneurysm and thereby promoting thrombosis.
The new device is indicated for endovascular treatment of adults with saccular wide-neck bifurcation intracranial aneurysms with dome diameters of 3 to 10 mm and either neck size ≥ 4 mm or a dome-to-neck ratio between 1 and 2.
Flow diverters offer a less invasive, more durable treatment for large intracranial aneurysms compared with coiling or open procedures. Pipeline™ and Surpass Streamline™ flow diverters were the first to be approved by the FDA. A third option — the Flow Re-Direction Endoluminal Device (FRED) — is in development.
“They represent a remarkable advance for previously untreatable aneurysms, allowing us to place a stent across the aneurysm in a short procedure,” says Dr. Bain. “The advent of these devices has been revolutionary.”
Advertisement
Dr. Bain was among the first in the U.S. to use the Surpass device, as detailed in this case study.
Diversion of blood flow occludes the aneurysm over months, reducing the risk of rupture (or re-rupture), with potential aneurysm disappearance within a year.
“The next 10 years will likely witness a continued explosion in these technologies that are enabling patients with previously untreatable aneurysms to look forward to normal, healthy lives,” says Dr. Bain.
Advertisement
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help