Researchers find evidence of a link in an animal model
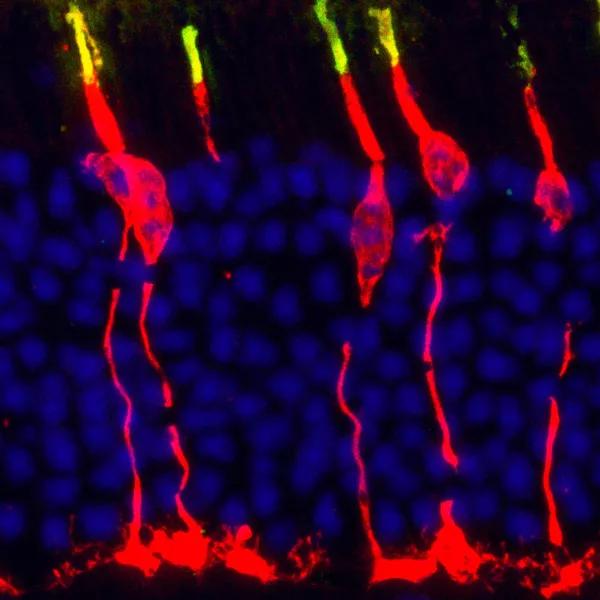
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/039ee70d-027c-4fc8-9444-d1ff7f805265/18-EYE-1117-Circadian-Rythems-CQD-Inset-1_jpg)
Circadian clock gene Bmal1 controls thyroid hormone-mediated spectral identity and function of cone photoreceptors. Cross section of a mouse retina labeled with a cone arrestin antibody (red) that marks the outer and inner segment, soma and synapse of the cone photoreceptor, while the short wavelength sensitive opsin protein (S-opsin) shown in green demarcates the cone outer segment. Mouse retina is rod dominated (blue nuclei) and less than 3 percent of photoreceptors are cones (red + blue nuclei).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Experts have long known that a person’s “circadian clock” can influence their metabolism, sleep patterns and immunity. In ophthalmology, circadian rhythms are known to regulate various aspects of photoreceptor physiology, but their contribution to photoreceptor development and function has been unclear.
Researchers at Cleveland Clinic’s Cole Eye Institute have now identified a link between the biological clock and photoreceptors in an animal model, and possibly to vision loss.
In a study published in Cell Reports, the researchers found that any disruption to the body’s natural circadian rhythm increases the risk of damage to cone photoreceptors.
“Overall, our results suggest a mechanism by which circadian proteins can locally regulate the availability of thyroid hormone (TH) and influence tissue development and function,” says Sujata Rao, PhD, senior author of the study.
“Several studies over the past 30 years have helped identify genes and proteins responsible for controlling the circadian clock,” she explains. “In our research, we discovered that these same genes and proteins also control the bioavailability of thyroid hormone in the retina and thus the development, function and aging of photoreceptors.”
Decoupling between the body’s internal clock and the thyroid hormone rhythms can trigger these photoreceptors to slowly deteriorate, which can potentially result in gradual vision loss or blindness.
For the study, researchers recorded electrical activity generated by the retina in response to various wavelengths of light from regular control mice and genetically modified mice lacking circadian clock genes. Using electroretinography (ERG), they found that control mice had normal electrical activity, while electrical activity in the genetically modified mice was lower.
Advertisement
Researchers also found that some defects caused by the disrupted clock could be partially reversed by providing the adult animals with the active form of thyroid hormone.
“This information is relevant because it tells us that we can activate the transcriptional machinery within the adult cone photoreceptors to turn on gene expression,” explains Onkar Sawant, PhD, co-author of the study.
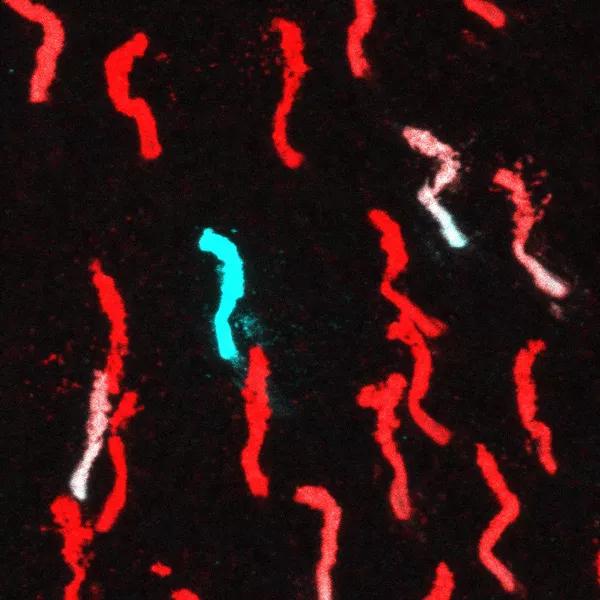
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9e21788a-71f6-4ea8-93f7-885c50201212/18-EYE-1117-Circadian-Rythems-CQD-Inset-2_jpg)
A snapshot image from the dorsal region of a mouse retina indicating the cone photopigments M-opsin (red) and S-opsin (cyan). Dorsal region of mouse retina is M-opsin enriched and very few S-opsin positive cones (cyan) or dual cones (white) are observed in this region. This distribution is altered when the circadian clock gene Bmal1 is deleted from the cone photoreceptors with all dorsal cones being converted to S-opsin expressing cones.
Nighttime light environment has changed dramatically due to modern technology, Dr. Rao points out, adding, “Increased usage of mobile devices during bedtime can disrupt your circadian clock. The intense light emitted from these devices has the potential to alter the timed release of factors within the eye, leading to chronic insults and susceptibility for visual damage.”
“Our findings suggest a functional role for the circadian clock in mammalian cone photoreceptor development and provide evidence for a continual role for TH signaling in cone photoreceptor maintenance,” Dr. Sawant continues. “Our findings may be of relevance in other tissues as well, where the circadian clock could alter tissue function by directly regulating enzyme type 2 iodothyronine deiodinase (Dio2) expression and thus TH signaling.”
Advertisement
With this new information, researchers can begin to ask questions such as, “How else can we change the photoreceptors?” and “Are there other factors that can improve photoreceptor function and help extend their viability?” Drs. Rao and Sawant say more research is needed.
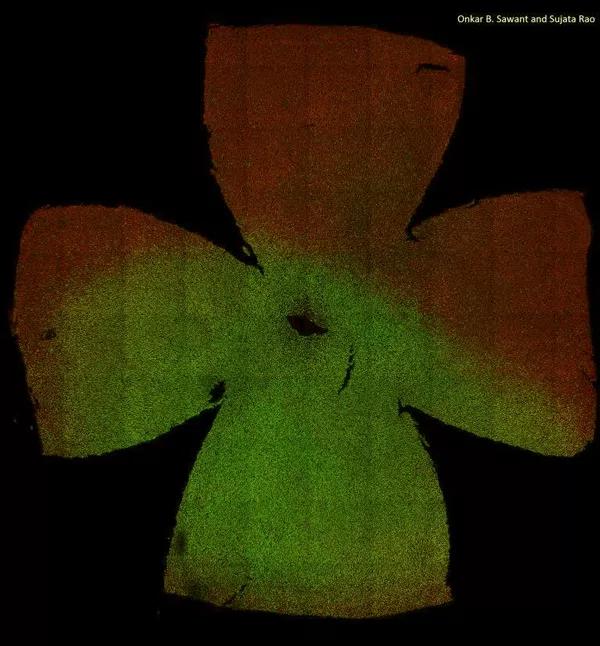
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/0cb91af5-ede2-4dc1-9d6c-7b522866cfb6/18-EYE-1117-Circadian-Rythems-CQD-Inset-3_jpg)
A composite image of the flat-mounted mouse retina, showing the spatial distribution of the cone photoreceptor subtypes. Retina is visualized using antibodies that mark the M-opsin (red) and S-opsin (green) proteins. Dorsal region of the mouse retina is M-opsin enriched and deprived of S-opsin. S-opsin cones are dominant in ventral retina. Deletion of circadian gene Bmal1 results in majority of the dorsal cones expressing S-opsin and thus disrupting the patterning of cone photoreceptor distribution.
Advertisement
Advertisement

Motion-tracking Brillouin microscopy pinpoints corneal weakness in the anterior stroma

Registry data highlight visual gains in patients with legal blindness

Prescribing eye drops is complicated by unknown risk of fetotoxicity and lack of clinical evidence

A look at emerging technology shaping retina surgery

A primer on MIGS methods and devices

7 keys to success for comprehensive ophthalmologists

Study is first to show reduction in autoimmune disease with the common diabetes and obesity drugs

Treatment options range from tetracycline injections to fat repositioning and cheek lift