Researchers to study retinal regeneration in zebrafish with new grant from National Eye Institute
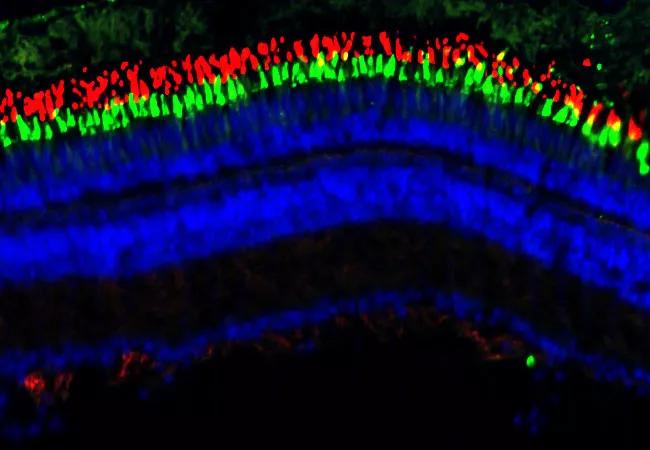
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/688f8b4c-ad46-4348-990b-f156403b5bbf/23-EYE-4196362-inflammation-retinal-regeneration-CQD-Hero_jpg)
23-EYE-4196362-inflammation-retinal-regeneration-CQD-Hero
Zebrafish have a remarkable ability to regenerate retinal neurons damaged by acute injury. Unlike humans, they can regrow photoreceptors and restore their visual function. Conventional belief is that mammals don’t have this ability. Retinal damage in humans, and the associated vision loss, is permanent.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We don’t know why photoreceptors don’t regenerate in humans,” says Brian Perkins, PhD, Vice Chair, Department of Ophthalmic Research, at Cleveland Clinic Cole Eye Institute. “Do human retinas completely lack the ability, or is the cellular machinery there but just not activated?”
There is some evidence that, with some tweaks to cellular processes, photoreceptors can be regenerated in mice — a first in mammals. That hints at the possibility for humans as well.
Dr. Perkins and his lab at Cleveland Clinic have been exploring this concept for more than a decade — building a foundation for potential retinal rewiring in humans. They hope to get one step further thanks to a new four-year research grant from the National Eye Institute, studying the role of inflammation in retinal regeneration in zebrafish.
In 2022, the Perkins team published a study that showed how the expression of one particular gene in zebrafish triggered retinal regeneration. When the gene was turned off, Müller glia cells in zebrafish retinas would reprogram and divide to produce neuronal progenitor cells, which would proliferate and differentiate into mature neurons.
But this only happened in the presence of inflammation. Preventing inflammation would prevent the process.
“There was a nuance to it,” explains Dr. Perkins. “There had to be a wave of initial inflammation that would soon get resolved. We learned that pro-inflammatory signaling was essential to initiate Müller glia proliferation, but resolving the inflammation was necessary for the survival and differentiation of neural precursors.”
Advertisement
In other words, to enable retinal regeneration in zebrafish, inflammation couldn’t be too little or too much, but just right. This may explain why zebrafish regenerate retinal neurons following acute injury but not in a model of chronic disease that produces long-term inflammation.
“The difference between injury and disease is not well understood, so that’s what we’re going to explore over the next four years,” says Dr. Perkins. “Our work could be valuable in the development of strategies to induce retinal regeneration in humans, who more commonly have retinal damage due to a chronic disease, not an acute injury.”
With collaborators David Hyde at the University of Notre Dame and Jiang Qian, PhD, at Johns Hopkins School of Medicine, Dr. Perkins and his research team will compare cell-level changes in healthy zebrafish to those in fish with a model of retinal degeneration, both before and after acute injury.
“Why do fish with disease not regenerate their retinas until after they’ve been injured? That’s one of the fundamental questions we will try to answer,” says Dr. Perkins. “We hope to gain insight into how disease is preventing the retina from regenerating in a species that is highly adapted to a regeneration process.”
Researchers will dissociate injured retinal cells from both healthy and diseased fish. Using next-generation sequencing with single-cell resolution, they will study what genes are being activated or inactivated at different time points after injury, as well as the activation of retinal stem cells and inflammatory cells.
Advertisement
From there, they hope to discover signaling pathways that could be manipulated with drugs or other mechanisms to promote regeneration in a diseased retina — potentially in mammals as well as fish.
Learning how chronic disease impacts retinal regeneration in a species that normally does regenerate may reveal new insights into retinal regeneration in humans with chronic retinal disease.
Today, patients with inherited retinal dystrophies typically progress to some level of permanent vision loss. While some gene therapies show great promise, they are targeted to specific mutations of specific genes. For the majority of people with an inherited retinal dystrophy, therapeutic options are limited.
The work by Dr. Perkins and his colleagues could lead to a more generic understanding of treating retinal dystrophy.
“Hopefully we can find ways to promote endogenous regeneration that could be applicable to all retinal dystrophies, not tailored to one particular disease, like a gene therapy,” he says. “Retinal regeneration in mammals hasn’t gotten as much attention as gene therapy, but there have been some amazing advances, including in mice, in the last couple of years. It’s just a matter of finding the right combination of ways to manipulate stem cells to activate, proliferate and turn into new photoreceptors. There’s a lot more promise than widely believed.”
Featured photo, above: An adult zebrafish retina is stained with markers to label the outer segments of rod photoreceptors (red) and cone photoreceptors (green). A stain for DNA labels the nucleus (blue) in all cells in the retina.
Advertisement
Advertisement
The advanced stage of diabetic retinopathy is among the most challenging for retinal surgeons
How to screen for and manage treatment-triggered uveitis
Leukocoria is the most common symptom of this rare childhood eye cancer
Unraveling the TNFA receptor 2/dendritic cell axis
Nasal bridge inflammation, ear swelling and neck stiffness narrow the differential diagnosis
Genetic testing at Cleveland Clinic provided patient with an updated diagnosis
Watch for sudden unilateral vision loss without pain
Preclinical study shows why it’s critical to consider sex in eye disease research