Innovative case provides guidance on when to consider this approach
Bridging to heart transplantation with Impella® pumps for both the left and right ventricles in a patient with severe biventricular heart failure is feasible, according to a first-in-world case report from Cleveland Clinic published in ESC Heart Failure (2019;6:552-554).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
While simultaneous use of right and left ventricular Impella devices in cardiogenic shock as a bridge to recovery or left ventricular assist device (LVAD) has previously been reported, this case marks the first published experience of their use in this fashion as a bridge to heart transplant.
“We have very few options for mechanical bridging to heart transplantation for patients with biventricular heart failure and cardiogenic shock,” says the lead cardiologist on the case, Antonio Perez, MD, MBA, Director of Cleveland Clinic’s Heart Failure Intensive Care Unit. “Our experience with this patient shows that two Impella devices can successfully be used simultaneously in this setting, providing an important new minimally invasive strategy.”
Many patients with cardiogenic shock from biventricular failure that requires acute mechanical circulatory support do not survive the time to transplantation, Dr. Perez explains. Durable VADs implanted into both ventricles have the advantages of high flow, durability and the possibility of patient ambulation, but this strategy requires sternotomy and prolonged intubation and carries risks of bleeding and mediastinal adhesions.
Minimally invasive options — including veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and use of biventricular percutaneous VADs such as the TandemHeart® and Impella devices — involve potentially less periprocedural morbidity and fewer operative risks, but VA-ECMO offers limited durability and all of these options preclude ambulation if femoral cannulation is required. The latter is an important limitation if the anticipated wait time to heart transplantation is long.
Advertisement
“For a patient expected to obtain a heart within a short period of time, avoiding the risks of surgical implantation of VADs or an artificial heart has multiple advantages,” notes cardiothoracic surgeon Edward Soltesz, MD, MPH, Surgical Director of Cleveland Clinic’s Kaufman Center for Heart Failure Treatment and Recovery, who served as surgical lead for this case.
The case patient, a 67-year-old woman presenting with chest discomfort, was diagnosed with complete heart block. Transthoracic echocardiogram (TTE) revealed mildly reduced left ventricular ejection fraction (LVEF) of 45%. No obstructive coronary disease was found by angiogram. She was discharged with a dual-chamber pacemaker.
Three weeks later, she returned with new heart failure symptoms. Now TTE showed LVEF of 15% with multiple segmental wall motion abnormalities. She also developed ventricular tachycardia and was treated with amiodarone and lidocaine infusions.
Cardiac MRI revealed marked thinning of the entire inter-ventricular septum and associated fibrosis and scarring with delayed contrast enhancement (Figure, A and B). Endomyocardial biopsy was deferred because of her clinical instability.
She was treated empirically with three days of methylprednisolone.
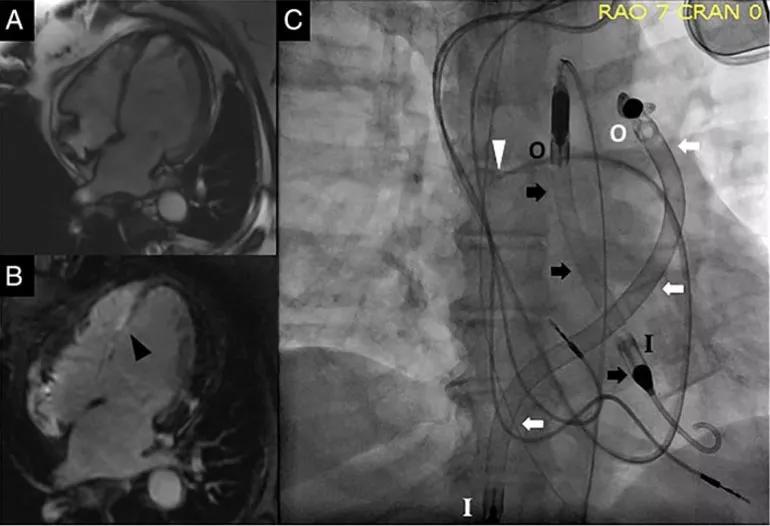
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/bcde5149-4bff-4c19-adbe-0192f76b271c/17-HRT-4808-double-Impella-770x526_jpg)
Figure. (A and B) Cardiac MRIs showing thinning of the inter-ventricular septum (A) and fibrosis/scarring of the inter-ventricular septum (B; black arrowhead). (C) Fluoroscopic images showing the pulmonary artery catheter (white arrowhead), the Impella 5.0 (black arrows) and the Impella RP (white arrows). I = inlet and O = outlet for the respective Impella devices. Reprinted from Varian et al., ESC Heart Failure (2019;6:552-554), ©2019 The authors.
Advertisement
An intra-arterial balloon pump (IABP) was inserted for cardiogenic shock and recurrent ventricular tachycardia. Her cardiac index remained low (1.5 L/min/m2) despite support.
An Impella 5.0® was placed femorally via surgical cut-down (an initial attempt via axillary approach failed because of small artery size). However, she continued to decompensate, with TTE revealing a newly dilated right ventricle with septal shift to the left.
An Impella RP® was then inserted percutaneously via the right femoral vein. With the Impella 5.0 set to deliver flow of 4.0 to 4.5 L/min, and the Impella RP delivering 4.0 L/min (Figure, C), cardiac index improved to 2.4 L/min/m2.
The patient developed severe vasoplegia, which was successfully treated with methylene blue. She continued to receive intravenous diuresis and was extubated after five days of biventricular mechanical support. She remained bedbound because of the femoral biventricular Impella cannulation. There were no device complications, and she required no blood transfusions.
On day 20, she underwent heart transplantation with surgical decannulation of the Impella devices. Histology of the heart revealed biventricular cardiac sarcoidosis.
Three weeks after transplantation, she was discharged to inpatient rehabilitation, after which she returned home. One year later, she is thriving and has normal functional capacity.
This patient’s successful course has led Cleveland Clinic’s cardiovascular team to adopt the following approach to mechanical bridging to heart transplant in patients with cardiogenic shock refractory to pharmacotherapy:
Advertisement
“Patient selection is critical to successful bridging to heart transplantation with biventricular mechanical circulatory support,” emphasizes Dr. Soltesz.
He explains that the case patient’s small body size (surface area 1.9 m2), common blood type (A) and low panel-reactive antibody score (0%) made her anticipated time to donor heart availability short, which qualified her as a good candidate for this strategy.
“In the right patient, biventricular support with Impella devices minimizes the risk of complications and reduces recovery time,” he adds. “These are goals that we are always striving for.”
Advertisement
Advertisement

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients