Researcher gives guidance for clinical trials
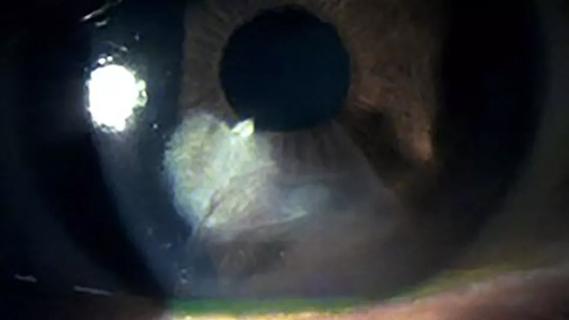
A 36-year-old woman developed severe diffuse lamellar keratitis of a LASIK flap, which resulted in corneal haze. Two months after surgery, she was referred for treatment of severe scarring fibrosis and uncorrected visual acuity of 20/200 (best-corrected visual acuity 20/30). Her consulting physician suggested off-label treatment with losartan, an angiotensin II receptor blocker (ARB), in the form of eye drops (0.8 mg/mL six times per day). Within five months, the patient’s uncorrected visual acuity had improved to 20/30 (best-corrected visual acuity 20/25), and the corneal haze was significantly reduced, based on slit lamp exam, Scheimpflug tomography and anterior segment optical coherence tomography (OCT).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This case study, published in November 2022 in the Journal of Refractive Surgery, is the beginning of what Cleveland Clinic refractive and corneal surgeon Steven E. Wilson, MD, hopes will be a rising tide of clinical studies on treating scarring corneal fibrosis with topical losartan. The drug commonly is used orally to treat hypertension.
Two studies published in 2022 by Dr. Wilson’s research team — one in Experimental Eye Research that showed clearing of corneal scarring after descemetorhexis, and one in Translational Vision Science & Technology that showed clearing of corneal scarring after alkali burn — revealed the dramatic effects of topical losartan in rabbit corneas. Dr. Wilson’s former research fellows, now practicing ophthalmologists in Brazil, applied the concepts to their patients with corneal scarring fibrosis. They used losartan off-label and produced their own topical solutions. The outcomes of their studies, including the case above, have been promising.
“Ophthalmologists in the U.S. also have begun treating patients with losartan off-label, in topical form,” says Dr. Wilson, Director of Corneal Research at Cleveland Clinic Cole Eye Institute. “Clinical studies are needed to learn more about dosage and treatment duration. It’s important to know that oral losartan taken by many patients does not penetrate the cornea and, therefore, has no effect on corneal wound healing and the fibrosis response to injury.”
To inspire more clinical trials, Dr. Wilson recently published in the Journal of Ocular Pharmacology and Therapeutics a list of ocular conditions that potentially could be treated with topical losartan, along with practical guidelines for using it.
Advertisement
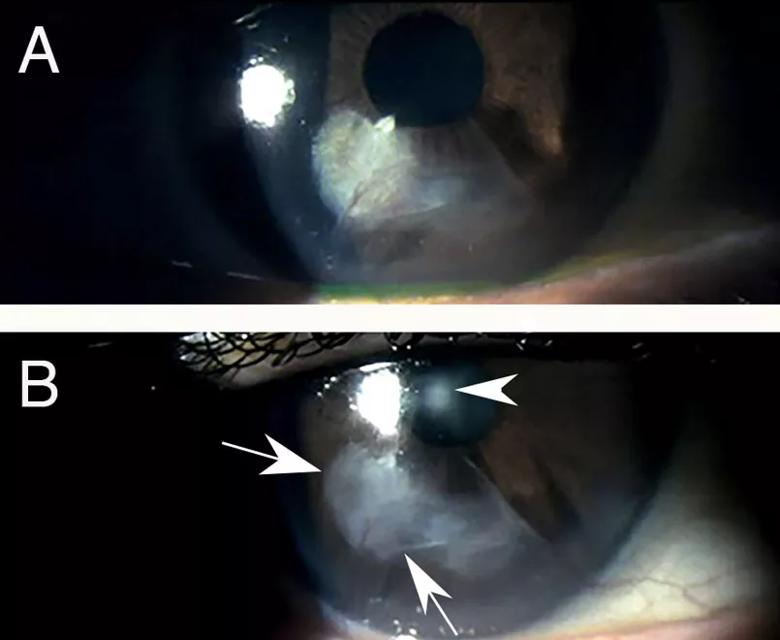
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5ff386cf-cf65-4a5f-ac10-83bd63961778/23-EYE-3521609-CQD-Medical-treatments-for-corneal-scarring-Wilson-inset_jpg)
Topical losartan treatment of progressive fibrosis in a radial keratotomy incision. A. Years after radial keratotomy, a patient reported decreasing uncorrected and best-corrected vision. The patient’s cornea was found to have developed progressive fibrosis within and surrounding an inferior incision. B. After one drop of 0.8 mg/mL losartan in balanced salt solution six times per day for 15 days, there was already a notable decrease in scarring fibrosis (arrows) and improvement in both uncorrected and best spectacle-corrected vision in the eye. The arrowhead is an artifact related to the light reflex during photography. Images provided by the treating physicians Rodrigo Carlos de Oliveira, MD, and Luiz Antônio Vieira, MD, São Paulo, Brazil. Mag. 15X. Reprinted with permission from the Journal of Ocular Pharmacology and Therapeutics. doi:10.1089/jop.2022.0174.
For three decades, Dr. Wilson’s lab has been funded by the National Institutes of Health and/or Department of Defense to study corneal wound healing. A focus of their research has been controlling scar-causing myofibroblasts, the main type of cell that causes corneal opacity after injury, infection and some surgeries. Myofibroblasts develop during a process driven by two growth factors: transforming growth factor (TGF) beta-1 and TGF beta-2. Losartan is known to hinder TGF beta signaling by inhibiting a signal transduction molecule, extracellular signal-regulated kinase (ERK).
“Topical losartan likely can prevent or reverse scarring anywhere myofibroblasts develop in the anterior segment of the eye, including the conjunctiva in fibrotic diseases of that tissue,” says Dr. Wilson.
Losartan is not expected to affect corneal fibroblasts, another type of cell that can cause corneal haze after photorefractive keratectomy (PRK) or corneal cross-linking, for example. That type of scarring is transient, disappearing with or without the use of steroids, and generally mild.
“More severe, persistent scarring is almost always caused by myofibroblasts,” says Dr. Wilson. “However, those cells are continually dying by apoptosis and being replaced with new myofibroblasts in scars in the cornea, even years after the original injury. If we interrupt the process, with losartan, for example, myofibroblasts that are dependent on TGF beta for generation and survival will instead be replaced with new, normal corneal cells that restore corneal transparency.”
Advertisement
Dr. Wilson’s research has led Cleveland Clinic to submit a patent on the use of topical losartan and other ARBs to prevent or reduce corneal scarring.
What eye diseases and disorders could be treated with losartan? Dr. Wilson’s article in the Journal of Ocular Pharmacology and Therapeutics includes a table of conditions that should be explored, including:
“We should study any disorder in which TGF beta is involved in the pathophysiology,” says Dr. Wilson. “Currently there are no good medical treatments for many of these conditions. Corticosteroids, for example, often are minimally effective in treating scarring fibrosis in these disorders, and their long-term use commonly is complicated by cataract, glaucoma or infections.”
The table also references adjuvant treatments, recommending when to combine losartan with corticosteroids, antibiotics or antivirals, for example.
Losartan dosing suggestions and precautions are noted throughout the article. Dr. Wilson advises investigators to limit concentrations of losartan to no more than 0.8 mg/mL in a balanced salt solution (pH 6.7-7.0) because different formulations have not been tested in animal models.
Advertisement
“Higher doses could interfere with normal TGF beta functions and lead to unforeseen complications,” he says. “We need to proceed with caution until higher dosages are carefully evaluated.”
It may take four to six months to gauge efficacy of losartan treatment in a particular patient, he adds.
“Topical losartan is the first therapy that has shown promise in clearing corneas with scarring fibrosis no matter how long-term,” says Dr. Wilson. “All research points to its effectiveness. We need more investigations — with Investigational New Drug and Institutional Review Board approvals, as appropriate — to explore the efficacy, safety and limitations in various disease states. The goal is to begin offering this promising therapy to more patients, some who have been managing vision deficits for years.”
While ocular losartan research to date has focused on anterior segment conditions, there is potential for posterior fibrotic diseases as well.
“I suspect losartan could be effective in treating conditions such as proliferative vitreoretinopathy and other diseases of the retina, choroid, vitreous and optic nerve, especially if administered with an extended-release intraocular device,” says Dr. Wilson. “We likely will uncover more possible applications as we continue our research.”
Advertisement
Advertisement

Motion-tracking Brillouin microscopy pinpoints corneal weakness in the anterior stroma

Study reveals more about the pathophysiology of Salzmann’s nodular degeneration
Studies continue to indicate effectiveness and safety

Identifies weak spots in the cornea before shape change occurs
Novel use of tPA reduces fibrin response in the eye

Untreated seropositive erosive RA led to peripheral ulcerative keratitis

Registry data highlight visual gains in patients with legal blindness

Prescribing eye drops is complicated by unknown risk of fetotoxicity and lack of clinical evidence